Recent breakthroughs suggest new ways to prevent, diagnose, and treat brain disorders associated with aging, including Alzheimer’s disease and related dementias
Alzheimer’s disease (AD) is a grand challenge. It’s common, affecting about 10% of the population; it’s expensive, costing the nation nearly $350 billion in 2023; and it often causes years of suffering for patients and their loved ones [1]. Other age-related neurodegenerative conditions, such as vascular dementia and Parkinson’s disease, also continue to pose problems, both for patients and society as a whole.
For those reasons, researchers from around the world are channeling significant energy into understanding—and ultimately slowing down—aging in the brain. New findings at the cellular level, including insights about myelin, mitochondria, and the blood–brain barrier (BBB), are offering clarity about when and why things start to go wrong. Meanwhile, promising new ways to detect dementia in its early stages, such as with a blood-based biomarker or by using GPT-3, could allow patients to get help sooner.
“The focus on this is considerable worldwide. It’s something that has everybody’s attention,” said Arthur W. Toga, Ph.D., director of the Mark and Mary Stevens Neuroimaging and Informatics Institute at the University of Southern California (USC). “That also makes it one of the more exciting opportunities for accelerating discovery in neuroscience, because we now have scientists from around the world cooperating to achieve a common goal.”
Unpacking cellular mechanisms of brain aging
After decades of research on AD, researchers are beginning to home in on the biological underpinnings of the disorder. The focus has long been on the patterns, frequency, and trajectory of two misfolded proteins, beta-amyloid and tau, that accumulate in the brain—both in AD and in several other disorders that cause dementia. Because those proteins are created and cleared naturally in a healthy brain, researchers are searching for dysregulated processes that may lead to the toxic buildup that occurs in dementia.
At USC, Toga and Berislav Zlokovic, MD, Ph.D., director of the Zilkha Neurogenetic Institute, have found that problems with the brain’s blood vessels—and the BBB in particular—are a hallmark of AD [2]. Leaks in the BBB, which is responsible for regulating what substances can enter and leave the brain, cause cellular damage in mice that then leads to cognitive decline (Figure 1).
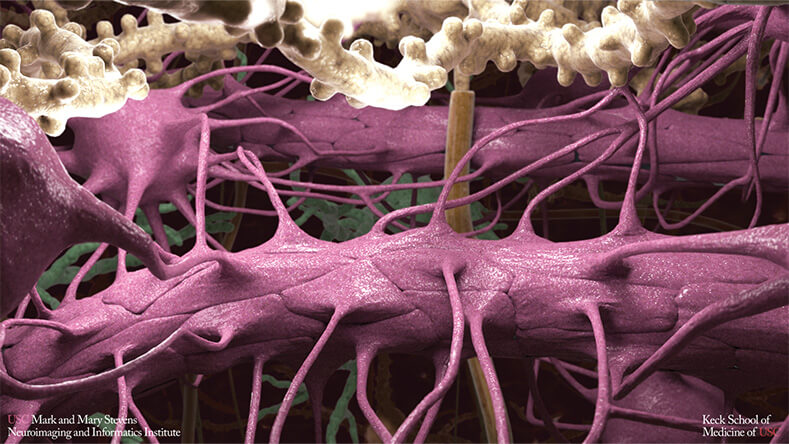
Figure 1. BBB determines what substances can enter and leave the brain. Research has linked its deterioration to the cellular damage and cognitive decline that occurs in Alzheimer’s disease. (Image courtesy of the USC Mark and Mary Stevens Neuroimaging and Informatics Institute.)
Comparing mice with a genetic risk for AD to those without, the team detected early damage to endothelial cells, which help control what can enter and exit the brain, and pericytes, which help keep the BBB healthy. Two to five months later, synaptic signaling between cells in certain brain regions showed signs of disruption. Next, behavioral changes began—mice performed worse on several tasks, indicating that memory loss had occurred [3].
“These findings point to the notion that vascular contributions to cognitive decline may be extremely important, because they might represent an opportunity for early intervention,” Toga said (Figure 2).
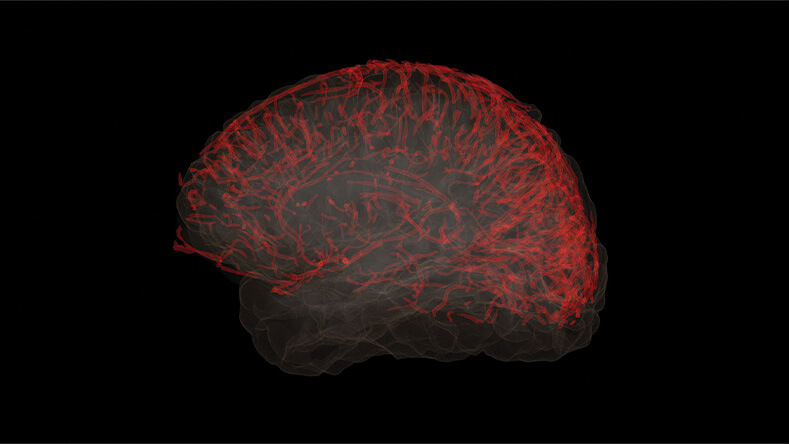
Figure 2. Recent findings suggest that the brain’s vascular system plays a significant role in the development of neurodegenerative diseases, including Alzheimer’s disease. (Image courtesy of the USC Mark and Mary Stevens Neuroimaging and Informatics Institute.)
An important cellular-level insight about AD relates to myelin, the insulating layer that surrounds axons and facilitates smooth communication between cells throughout the nervous system. At the Max Planck Institute for Multidisciplinary Sciences, Göttingen, Germany, Klaus-Armin Nave, Ph.D., and his colleagues discovered that mice with a myelinating disorder exhibited changes in the brain’s white matter very similar to those that occur in normal human brain aging, including structural changes in myelin and the degeneration of axons. So, Nave and his colleagues used those mice as a proxy for typical aging processes, crossbreeding them with AD mice to understand why aging increases the risk for AD [4].
“We found indeed that if you ‘stress’ an Alzheimer’s mouse model with premature white matter aging, it has more of this myelin-triggered Alzheimer’s pathology,” said Nave, who directs the Max Planck Institute. “That’s what’s new here: We know that these white matter changes in the aging mammalian brain are really a driving force of the Alzheimer’s pathology.”
They found that in both mouse and human models of AD, myelin in the brain’s cortex is lost and not replaced. That damaged myelin then becomes a distraction for glial cells—instead of clearing beta-amyloid, they focus on clearing myelin. Therapeutic efforts to replace damaged myelin with fresh myelin could therefore help slow the age-related brain changes that contribute to AD.
Another novel line of research suggests that damage to mitochondria over the lifespan could contribute to the buildup of toxic proteins linked with AD, Parkinson’s disease, and other brain disorders. Researchers from Hebrew University, Jerusalem, Israel, found that an injection of fresh mitochondria reversed cognitive defects and decreased brain cell death in AD mouse models [5], suggesting another promising therapeutic target.
Tools for early detection
If reversing cognitive decline is possible, early diagnosis will also be critical for giving patients the best shot at halting, reversing, or delaying the progression of the disease. For that reason, researchers are searching for ways to detect and diagnose AD during its earliest stages. To replace costly, time-consuming, and highly specialized tests for signs of AD (such as positron emission tomography (PET) scanning), several groups are working to find blood-based biomarkers for the disorder and to develop an associated blood test [6].
“Scanning doesn’t scale easily, so it’s not the best way for us to test large populations practically. But a plasma biomarker does,” Toga said. “Rather than wait for someone to show up in a clinic because they no longer remember where they live, we can screen people for tau deposition the same way we screen people for high cholesterol.”
Others are leveraging new technologies in clever ways that could ultimately allow dementia screenings to occur anytime, anywhere. Hualou Liang, Ph.D., a professor of biomedical engineering at Drexel University, Philadelphia, PA, USA, and his doctoral student Felix Agbavor tested whether Open AI’s GPT-3 could be used to detect differences in speech patterns between healthy older adults and those with dementia [7]. Artificial intelligence (AI) has been used to analyze speech in dementia patients before, but the new study is among the first to explore the potential of generative AI.
Agbavor and Liang [7] trained their model using transcripts from a dataset of speech recordings and then found that it could predict early cases of dementia with 80% accuracy. The model detected signs of impairment such as word repetition, grammatical errors, and increased use of filler words (such as “um” and “uh”). The researchers also tested whether the model can be used to predict Mini-Mental State Exam (MMSE) scores, which are used to estimate dementia severity. The new model was 20% more accurate at predicting MMSE scores than a model based solely on acoustic features of the recordings. Next, the researchers aim to commercialize the technology and create a mobile application that can be used at home, prompting those who need it to seek formal evaluation from a health care provider.
“If every older person who becomes forgetful goes to the clinic for cognitive testing, it’s time-consuming, expensive, and not a fit for a larger scale,” Liang said. “If we can develop this technique, purely based on the voice, people can do pre-screening at home. We think this has a huge potential to help people as they age.”
Unlocking new interventions
Looking forward, researchers across the field are actively seeking ways to broaden dementia research to include populations that are typically left out. Liang’s next big project is a collaboration with researchers at Thomas Jefferson University who will help him test his AI model among AD patients who are African American. At USC, Toga’s group has partnered with Sid O’Bryant, Ph.D., of the University of North Texas, to conduct a large-scale longitudinal study of AD (which involves collecting brain scans, blood samples, and neuropsychological tests over a five-year period) in African American and Latino older adults.
A better fundamental understanding of the aging process will also bolster existing efforts in the field, said Nave. For example, what processes drive epigenetic silencing, where cells accumulate genetic defects over time, and why does aging occur at different rates and junctures in different species? Answering those questions will help scientists continue to flesh out the granular details of aging and in turn find ways to slow it down.
“To some degree, efforts to treat dementia are not targeting the underlying biology of the disease, because we don’t yet fully understand the mechanism,” Toga said. “That’s one of the grand challenges the field faces—when that occurs, we’ll unlock a vast array of new interventions.”
References
- Alzheimer’s Association. Alzheimer’s Disease Facts and Figures. Accessed: Jun. 26, 2023. [Online]. Available: https://www.alz.org/alzheimers-dementia/facts-figures
- A. Montagne et al., “APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline,” Nature, vol. 581, no. 7806, pp. 71–76, May 2020, doi: 10.1038/s41586-020-2247-3.
- G. Barisano et al., “A ‘multi-omics’ analysis of blood–brain barrier and synaptic dysfunction in APOE4 mice,” J. Exp. Med., vol. 219, no. 11, Aug. 2022, Art. no. e20221137, doi: 10.1084/jem.20221137.
- C. Depp et al., “Myelin dysfunction drives amyloid-β deposition in models of Alzheimer’s disease,” Nature, vol. 618, no. 7964, pp. 349–357, May 2023, doi: 10.1038/s41586-023-06120-6.
- K. Nitzan et al., “Mitochondrial transfer ameliorates cognitive deficits, neuronal loss, and gliosis in Alzheimer’s disease mice,” J. Alzheimer’s Disease, vol. 72, no. 2, pp. 587–604, Nov. 2019, doi: 10.3233/JAD-190853.
- F. Gonzalez-Ortiz et al., “Brain-derived tau: A novel blood-based biomarker for Alzheimer’s disease-type neurodegeneration,” Brain, vol. 146, no. 3, pp. 1152–1165, Mar. 2023, doi: 10.1093/brain/awac407.
- F. Agbavor and H. Liang, “Predicting dementia from spontaneous speech using large language models,” PLOS Digit. Health, vol. 1, no. 12, Dec. 2022, Art. no. e0000168, doi: 10.1371/journal.pdig.0000168.



