This is the fourth in a series of articles on the dramatic transformation taking place in health informatics in large part because of the new Health Level 7 (HL7) Fast Healthcare Interoperability Resources (FHIR) standard. The first article provided the background on healthcare, electronic health record systems for physicians, and the challenges they both face along with the potential of interoperability to help overcome them. The second introduced the basics of the FHIR standard and some suggested resources for those who are interested in its further exploration. The third introduced SMART on FHIR, which, based on its wide adoption, has become the default standard FHIR app platform. This article introduces clinical decision support as an important use case for FHIR in support of health care providers. The articles in this series are intended to introduce researchers from other fields to this one and assume no prior knowledge of health care or health informatics. They are abstracted from the author’s book Health Informatics on FHIR: How HL7’s New API is Transforming Healthcare (Springer International Publishing).
Clinical Decision Support (CDS) is an early, important, and particularly interesting domain within health informatics. Its purpose is to provide clinicians, patients, and others with knowledge and personalized information, intelligently filtered or presented at appropriate times, to enhance health and health care. It is a critical component of the IOM’s vision of a Learning Health System that we introduced in the first article since it is the vehicle for feeding knowledge obtained from the care of prior patients back to providers caring for current patients or even directly to those patients.
According to the Office of the National Coordinator for Health Information Technology (ONC), “CDS tools include computerized alerts and reminders to care providers and patients; clinical guidelines; condition-specific order sets; focused patient data reports and summaries; documentation templates; diagnostic support, and contextually relevant reference information, among other tools.”
CDS was one of the first applications of computing to health care. Beginning in the mid-1950s, Dr. Homer Warner, a health informatics pioneer, began using computers for decision support in cardiology at LDS Hospital (now Intermountain Healthcare) in Salt Lake City. The Health Education through Logical Processing (HELP) system’s knowledge-based decision processor received data from virtually all areas of the hospital to provide a variety of CDS tools. Dr. Ted Shortliffe, a Stanford-based pioneer in the field, developed MYCIN to assist physicians in antibiotic therapy in the early 1970s. MYCIN was an early backward chaining expert system. This technique starts with the goal (is there an antibiotic whose use is supported by the clinical evidence) and works back to the available data to see if it provides the necessary support for a clinical recommendation. MYCIN also provided an appropriate dosage adjusted for patients’ body weight. INTERNIST was an arguably even more ambitious early CDS system developed, starting in 1974, at the University of Pittsburgh by Dr. Jack Myers, an internal medicine physician, and Dr. Harry E. Pople, a computer scientist and pioneer in artificial intelligence. The goal was to make the most appropriate diagnosis in a given clinical situation. Health informatics is not often the first place a new computer science technique appears but, according to volume 64 of the Encyclopedia of Library and Information Science, “Applying Abduction to artificial intelligence problems began with Harry Pople and his system, INTERNIST.”
Despite the early recognition of the potential of CDS, it failed to achieve widespread acceptance in large part due to two issues that are now finally being addressed by Fast Healthcare Interoperability Resources (FHIR) and SMART on FHIR apps. The first is duplicate data entry. All early CDS systems preceded electronic medical record systems or were independently developed software. In either case, using them meant inputting clinical data that had already been recorded, most likely on paper. Unsurprisingly, busy physicians did not find this very appealing. The second issue was that these systems were “off to the side” running on large, heavy devices and did not merge well into the workflows and processes of clinical practice. To some degree, modern mobile computer hardware helps resolve this issue but a full resolution requires that CDS systems be an integral component of charting in an electronic health record. We saw in the third article how the RIMIDI SMART on FHIR app can be so tightly integrated with an EHR (Cerner’s PowerChart in the example) that for all practical purposes it appears to the physician to be part of charting which substantially solves the workflow issue. We also saw how launch parameters can automatically fetch the patient-specific data needed to do the CDS resolving the dual data entry problem.
A Case Study in Intensive Care
Before we consider a current example of state-of-the-art CDS using SMART on FHIR we should touch upon the fundamental role that machine learning is playing in developing these tools. The early CDS systems we discussed were rule-based. Typically, the earlier rules derived from experts in a particular domain and were at the heart of the CDS system. For example, INTERNIST contained a knowledge base consisting of some 500 disease entities, organized into categories by organ system, and their over 3,000 clinical manifestations. I can still recall visiting Dr. Homer Warner and seeing the highly structured methodology he had developed to derive clinical rules from expert panels.
Today, the goal is often prediction, and machine learning is employed to derive the features that are most predictive of the specific outcome under consideration. Here we will discuss Emory School of Medicine’s Artificial Intelligence Sepsis Expert (AISE) and its incorporation into an FHIR app called DeepAISE on FHIR [1].
Sepsis is a life-threatening condition that arises when the body’s response to infection injures its own tissues, including major organs. It is currently one of the important problems in medicine due to its complexity from pathophysiologic, clinical, and therapeutic viewpoints. Sepsis is an important cause of hospitalization and is a particularly common problem in intensive care units (ICU). Affecting about 25% of admitted patients, it is the leading cause of deaths among patients in noncoronary care ICUs. The Healthcare Cost and Utilization Project (HCUP) and the Agency for Healthcare Research and Quality (AHRQ) place the 2013 annual cost of hospital care for sepsis patients in the United States at US $24 billion.
Given its complexity, sepsis can be difficult to diagnose, and early diagnosis is critical, as demonstrated by a large 2006 study that found that “[e]ach hour of delay in antimicrobial administration over the ensuing 6 hours was associated with an average decrease in survival of 7.6%” [2].
The goal of AISE is earlier sepsis diagnosis. Its expert engine was trained on 31,000 Emory ICU patients and validated on 52,000 patients from MIMIC-III, a freely accessible critical care database developed at the MIT Laboratory for Computational Physiology [3].
Given its complexity and subtlety, the definition of sepsis has changed over the years. AISE is based on the Third International Consensus Definitions for Sepsis (Sepsis-3). The research team has concluded that the tool can predict sepsis 4 hours in advance (ROC of 0.85).
As with all CDS, integration into workflow and process is a critical factor for acceptance. The ICU is a particularly challenging environment for patient care and for CDS. ICUs manage extremely sick patients, depend on a rich variety of data to monitor them, and patient status can change quite rapidly. At the same time, the resident staff’s attention is often focused on one or more particularly sick patients. The electronic ICU (eICU), a form of telemedicine that uses state-of-the-art technology to provide an additional layer of critical care service, was developed to help assure that nothing gets missed in this complex, dynamic environment. The eICU is staffed by skilled nurses and physicians with years of ICU experience. Given their oversight role, AISE is currently being prepared for a clinical trial in eICUs located in Atlanta, Georgia, and Royal Perth Hospital in Western Australia.
DeepAISE on FHIR provides an excellent example of how SMART on FHIR apps can integrate into workflow and how FHIR data streams can avoid the need for duplicate data entry. The model uses 65 clinical features (variables) that are updated and used to recalculate the prediction on an hourly basis. Since the eICU system currently in use is not yet FHIR-enabled, the data needed for DeepAISE is queried hourly and moved to the Google Health Cloud where it is stored in an FHIR store, and is subsequently analyzed by the AISE model, and its predictions and the underlying clinical data are made available to the Deep AISE app via FHIR.
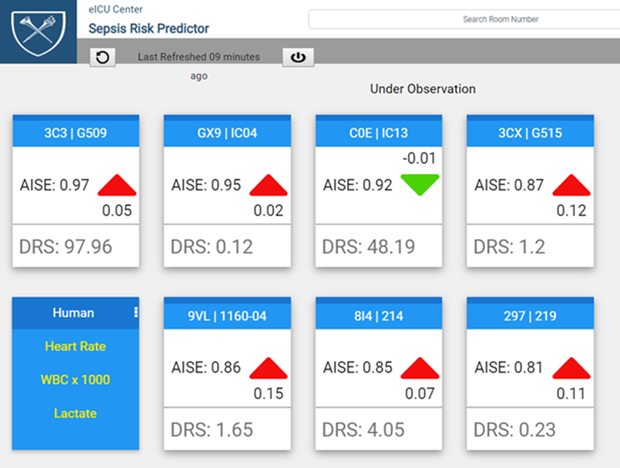
The transfer of the underlying data to the AISE tool is another important requirement for CDS acceptance. Physicians will not generally accept a computer-based recommendation unless the clinical basis for that advice is clear. Figure 1 illustrates how DeepAISE on FHIR accomplishes this and the resultant importance of having both the model’s risk scores as well as the underlying clinical data continuously available.
In this article, we looked in more detail at how FHIR can provide valuable tools to clinicians. In the next article, we will take a closer look at how FHIR apps are being used by their patients.
References
- S. Nemati, A. Holder, F. Razmi, M. D. Stanley, G. D. Clifford, and T. G. Buchman, “An interpretable machine learning model for accurate prediction of sepsis in the ICU,” Crit. Care Med., vol. 46, no. 4, pp. 547–553, Apr. 2018.
- A. Kumar et al., “Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock,” Crit. Care Med., vol. 34, no. 6, pp. 1589–1596, Jun. 2006.
- MIMIC Critical Care Database. (2015). [Online].



