Researchers develop new biocompatible and customizable materials to use in medical devices, implants, and drug delivery systems
Plastic has done more to revolutionize the medical industry over the past century than any other material. Syringes, intravenous bags, personal protective equipment (PPE), catheters, and test kits—plastic is ubiquitous throughout medicine. It’s easy to see why. Plastic is low-cost, easy to process, and can be sterilized efficiently.
But fossil-fuel-based plastics make up roughly one-quarter of the 6 million tons of medical waste generated in the U.S. alone each year; less than 10% of that is recycled. The bulk of the remainder is made up of single-use materials tossed out to prevent the spread of infection. The COVID-19 pandemic brought PPE—masks, gowns, gloves, head and shoe covers, face shields, and goggles—into the public consciousness as it created a global spike in the production and disposal of this single-use, largely plastic gear.
A 2019 report in the journal Environmental Science and Technology estimated that Americans ingest and inhale 74,000–121,000 microplastic particles every year, with unknown health consequences [1]. And with the growing cacophony of reports about environmental damage caused both by the waste plastic that creates those particles and by the fossil fuels used to produce plastic in the first place, health care providers are asking: Does the profligate use of plastic conflict with the medical credo “do no harm”? The industry isn’t alone in its concern. With worldwide production of plastic expected to exceed 600 million tons by 2030, no one can afford to turn a blind eye to the problem.
Enter bioplastics. Made using renewable biological sources that range from vegetable oil and food waste to sawdust and micro-organisms, biomass polymers increasingly are competing on both cost and scale against plastics made using fossil fuels. Bioplastics manufacturers are scaling production rapidly; analysts at IDTechEx expect the industry to grow by 10.1% annually over the next decade to more than 7 million metric tons by 2033 [2]. While bioplastics ultimately could help the medical sector become more sustainable, they’re also of interest to researchers seeking new biocompatible and customizable materials to use in medical devices, implants, and drug delivery systems. An added bonus: Several bioplastic source materials have natural antimicrobial properties.
Converting waste into biopolymers
Bioplastics have been used for years in a number of medical channels, including for drug delivery via gelatin-based capsules, biodegradable stitches, and bio-based bandages to promote blood clotting and skin regeneration. And researchers now are seeking new ways to ramp up bioplastic production, potentially stimulating novel uses.
“The biopolymers that we’re working with either have been approved by the FDA already or they’re GRAS—generally regarded as safe,” explains Joshua Yuan, who has developed several novel bioplastic processes initially at Texas A&M University and now at Washington University in St. Louis. “We already know that they are not toxic, so we don’t have to go through the lengthy FDA approval process.”
Yuan’s research teams have focused on producing bioplastics more cheaply and efficiently (Figure 1). Initially, they worked to harness a waste byproduct from wood pulp called lignin. More recently, the teams have been using carbon dioxide as a feedstock for bacteria used to produce bioplastics.
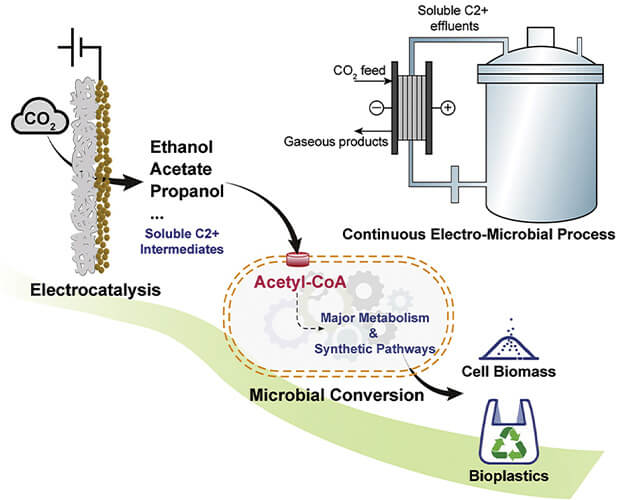
Figure 1. Chem–bio interface design for rapid conversion of CO2 to bioplastics in an integrated system. (Image courtesy of Texas A&M University and Washington University in St. Louis.)
Today’s biorefineries that produce ethanol yield lignin as a waste byproduct, much of which is burned off. Yuan’s team worked to convert that lignin into a valuable resource itself. They created a “plug-in” preconditioning process—designed as an inexpensive retrofit—that integrates five conventional pretreatments to dissolve, condition, and ferment lignin [3]. The process produces both ethanol and a type of bioplastic called polyhydroxyalkanoate, or PHA, at the same time. By promoting lignin’s use to produce bioplastics, the new process improves biorefinery economics while reducing the need to grow separate crops to make bioplastics.
The team subsequently released a paper describing how carbon dioxide could be converted into PHA through a process involving electrocatalysis and a unique chemical–biological interface with bacteria [4]. “In theory, it is kind of like a train with units connected to each other,” said Susie Dai, an associate professor of plant pathology and microbiology at Texas A&M who collaborated with Yuan. “The first unit uses electricity to convert the carbon dioxide to ethanol and other two-carbon molecules—a process called electrocatalysis. In the second unit, the bacteria consume the ethanol and carbon molecules to become a machine to produce bioplastics.”
PHAs, which can be used in medical applications ranging from bedpans and urine bottles to sterile single-use packaging and drug delivery agents, are completely degradable bioplastics. Two decades ago, PHAs cost as much as $20 per kilogram, but as production capacity grows, they can be made for less than $3 per kilogram.
Bioplastics move inside the body
In addition to such large-scale production uses, PHAs also can be useful for biocompatible tissue replacements and devices used inside the human body, said Neel Joshi, whose lab at Northeastern University is working with engineered biofilms called aquaplastic.
“While we mostly think of PHAs in the context of packaging and single-use plastics, they can be designed to degrade inside a body, which many biopolymers will do,” he said. “So bioplastic use in the medical field has two different motivations: The plastic waste issue and the development of new biomedical applications.”
Joshi’s work focuses on the latter motivation. Through genetic engineering of Escherichia coli, or E. coli, his lab has created a new class of degradable and biocompatible plastic that self-assembles into fibers that can be dried at room temperature into flexible sheets of bioplastic film. The sheets subsequently can be molded in water into three-dimensional shapes or thinned to use as a coating as shown in Figure 2. “It’s plastic-like, but completely made of biological materials,” he said [5].
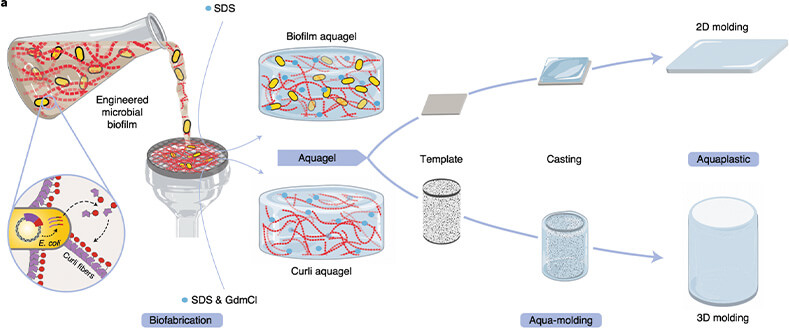
Figure 2. Fabrication of aquaplastic directly from engineered microbial biofilms. (Image courtesy of the Joshi Lab, Northeastern University.)
Other researchers are focusing their efforts on novel materials that use the combined strengths of biopolymers and the body’s natural processes to create regenerative medicine treatments. At Cornell University, four research labs from three different departments collaborated to develop a biohybrid material from collagen and hydrogel that mimics natural tissue (Figure 3). The ultimate goal: To replace and regrow worn cartilage in knees, elbows, and other joints [6].
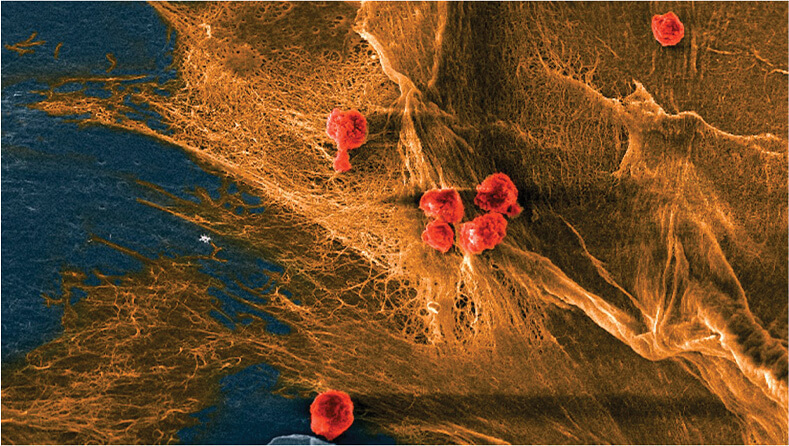
Figure 3. Micrograph of a biohybrid composite material developed at Cornell shows cells (red) seeded on the fibrous domains (yellow) of collagen. The material mimics natural tissue in its softness, toughness, and ability to recruit cells and keep them alive. (Image courtesy of Bouklas Lab, Cornell.)
“This quest for the best materials to house cartilage cells for tissue regeneration has been going on for a couple of decades,” said Lawrence Bonassar, co-lead author of a paper describing the work and a professor in both biomedical and mechanical engineering at Cornell. “You can find materials that are cell-compatible but don’t have the right mechanical properties, while the right mechanical properties won’t be particularly cell-compatible. None of us alone could have ever gotten here.”
The base material for the biohybrid material—collagen—is the body’s primary structural protein, Bonassar explained. Previous efforts to use collagen as a biomaterial have failed because the naturally pliable material becomes too brittle when anything is added to enhance its durability.
But the Cornell team instead mixed collagen with a zwitterionic hydrogel containing both positively and negatively charged molecular groups. The hydrogel interacts with charged groups in the collagen, self-assembling into the same interconnected network of collagen seen in natural cartilage, which inherently toughens the material. When the team seeded the biohybrid substance with cartilage, the cells attached themselves to the material’s networks.
“Ultimately we want to create something for regenerative medicine purposes, such as a piece of scaffold that can withstand some initial loads until the tissue fully regenerates,” said Nikolaos Bouklas, also a co-lead author of the paper. “With this material, you could 3D print a porous scaffold with cells that could eventually create the actual tissue around the scaffold.”
Combined with efforts to substitute bioplastics for inert medical plastic waste, such innovations could go a long way toward restoring plastics’ reputation as a medical miracle.
References
- K. D. Cox et al. Human Consumption of Microplastics. Accessed: Feb. 8, 2023. [Online]. Available: https://pubs.acs.org/doi/10.1021/acs.est.9b01517
- A. Ko. Bioplastics 2023–2033: Technology, Market, Players, and Forecasts. Accessed: Nov. 14, 2022. [Online]. Available: https://www.idtechex.com/en/research-report/bioplastics-2023-2033-technology-market-players-and-forecasts/880
- Z. H. Liu et al. Transforming Biorefinery Designs With ‘Plug-In Processes of Lignin’ to Enable Economic Waste Valorization. Accessed: Nov. 18, 2022. [Online]. Available: https://www.nature.com/articles/s41467-021-23920-4
- P. Zhang et al. Chem-Bio Interface Design for Rapid Conversion of CO2 to Bioplastics in an Integrated System. Accessed: Nov. 18, 2022. [Online]. Available: https://www.sciencedirect.com/science/article/pii/S2451929422004788
- A. M. Duraj-Thatte et al. Water-Processable, Biodegradable and Coatable Aquaplastic From Engineered Biofilms. Accessed: Nov. 15, 2022. [Online]. Available: https://www.nature.com/articles/s41589-021-00773-y
- C. Darkes-Burkey et al. Simple Synthesis of Soft, Tough, and Cytocompatible Biohybrid Composites. Accessed: Nov. 15, 2022. [Online]. Available: https://www.pnas.org/doi/10.1073/pnas.2116675119



