The phrase “made in China” is everywhere, just not in our health care system. China ranks as the world’s third-largest medical device market and is expected to become the second-largest market in the world in five to seven years. However, almost all of China’s hospitals, not to mention its larger first-class hospitals, use imported devices. For a very long time, foreign-made products have dominated the middle- and high-end markets, leaving to Chinese companies the low-end products with low profit margins. According to recent statistics provided by the China Chamber of Commerce for Import and Export of Medicine and Health Products, 90% of value-added high-tech devices, which account for 70% of China’s medical device market, are imported. Why can’t Chinese companies compete with multinational biomedical corporations? Is it because of a lack of money or intelligence?
Innovation in the medical device industry has been identified as a top priority by the Chinese government. The eagerness and anxiety to accelerate the pace have spread across the nation, but actually spurring that innovation remains obscure. Scientists, entrepreneurs, and high-level government officials alike are making great efforts to change this dire situation. One such undertaking has been the founding of the Suzhou Institute of Biomedical Engineering and Technology (SIBET) located in the city of Suzhou in the Jiangsu Province in Eastern China (pictured above).
The Idea
Scientific discoveries and technological developments, when translated to industrial mass production, must go through painstaking engineering studies and amplification experiments to achieve suitable and stable results. Especially in the health care industry, which involves human lives, the scrutiny of new products must be particularly meticulous, and the road from research to clinical use can be extraordinarily long.
In China, research institutes and universities are the main force of technological advancement, but because of a lack of intrinsic motivation, external economic support, and social resources such as closely related capital investors, the percentage of inventions that go to mass production is less than 30%, according to studies conducted at the Jiangsu Information Institute of Science and Technology. Scholars mostly concentrate on the pioneering side of technology, but research at this stage shows a low degree of technological maturity. The intrinsic high risk in health care R&D severely limits its rapid promotion, resulting in a number of failed industrialization attempts. On the other hand, industry demands mature and low-risk technologies and is very reluctant to put money into projects that show low short-term return. Again, in the health care industry, it takes years of research and application to acquire a regulatory approval. Few companies have the patience to babysit seedlings; thus, their experience in finance and operation helps little when it comes to the translation from a laboratory into a company.
According to statistics provided by the China High-Tech Industrialization Association, the success rate in the industrialization of scientific research depends on the prototyping process. After successful prototyping, the industrial success rate increases up to 80%; without this process, success rates drop to less than 25%. However, prototyping is the weakest link in the Chinese health care industry. Funding of R&D, prototyping, and industrial production should stay at a reasonable ratio of 1:10:100. The current ratio in China is only 1:0.07:100, which is very low. Prototyping needs serious capital investment; otherwise, it will be the limiting factor in the industrialization of scientific and technological achievements and will ultimately break the entire industrial chain of innovation. R&D projects are generally funded by the government, while mature products have companies waiting in line to acquire them. This leaves high-risk and unpredictable projects unwanted and unfunded, which is exactly where we come in.
The Mission
SIBET, which began its translational platform only last year, was built with a clear mission—to fill the gap between research and industry. But how? How can we organize and streamline the process to revolutionize the health care industry? Unfortunately, nobody knows. SIBET is a fledgling experiment designed to explore a new and suitable way to optimize the whole translational process for China.
It is clear that Suzhou, China, is the ideal location for this experiment. Suzhou ranks number six in gross domestic product among all cities in China, and many high-tech health-care industrial companies are located here. It is very convenient for scientists to turn their ideas into reality, and local companies are also quick to absorb new ideas and technologies. It is also clear that the Chinese Academy of Sciences (CAS) is the ideal vessel for this experiment. CAS has the technological capabilities and know-how to develop a broad range of medical devices for the market. Through collaboration, the R&D process in the CAS system is efficient. With those two strong supports, SIBET has put all of its energy into the prototyping process.
The medical device industry is unique in that clinical trials and qualification are challenging and necessary for a company to advance a product into the market. The medical device industry is also internationally recognized as a high-tech industry, showing a high degree of innovation, integration, penetration, and competition. It also has a long conversion cycle and higher risk. The development of medical devices reflects a multidisciplinary, multitechnological integration as well as a fusion of basic research, applied research, development, and commercialization. This is why the traditional incubator model does not apply here. To survive in the health care business, in addition to the soft environment (policies) and the physical space commonly provided by the government, the more urgent need is for professional and technical personnel (an engineering platform) and funding support for projects. SIBET fits in perfectly as an intelligence pool and a technology pool, with all the supporting services in place. We are establishing a newer version of the incubator model. What sets this model apart is its focus on translation. SIBET is determined to stand shoulder to shoulder with world-class incubators such as Silicon Valley in the United States and Zhong Guan Cun in China. SIBET’s bureaucracy is limited to make things happen faster. We screen projects carefully and gather all of the resources needed to produce just a handful of great products at a time. It’s an innovation machine.
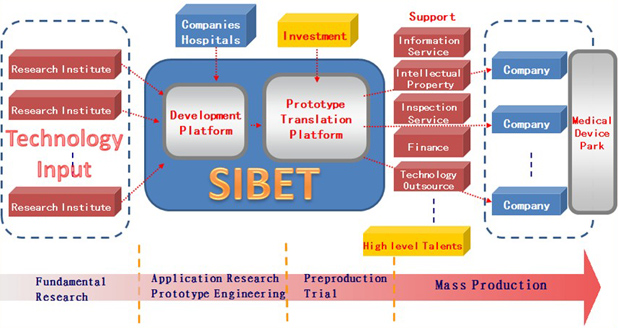
Simply put, SIBET’s main goal is to transform promising early ideas into well-polished products. Although our platform is still in its infancy, it has been carefully crafted to fill some critical gaps in bringing new and innovative ideas to the market. Here is how our model, above, has been developed:
- Input: This requires a global vision to find suitable projects. Any new ideas, basic research, and laboratory prototypes related to health care products are to be evaluated by SIBET. A few companies or agents should be involved in this phase. SIBET conducts a risk assessment that will discard ideas that prove to be unfeasible.
- Pre-production platform: This includes:
- A screening mechanism: To reduce the translational risk, SIBET will seek to identify where the risk is. Two committees (the Technology Committee and the Investment Committee) are responsible for screening translational projects from different perspectives. The Technology Committee aims to evaluate the feasibility and advancement of the technology. The Investment Committee aims to estimate the market value from an economic perspective. The Technology and Investment Committees are composed of academic scholars, engineers, medical doctors, venture capitalists, and entrepreneurs. Investment decisions are made by the board of directors according to the suggestions of the two committees.
- Funding resources: SIBET seeks to attract funding from government, venture capitalists, companies, and banks. Different resources play different roles. Government funding is dedicated to preproduction and is managed by SIBET as “seed” funding, which is designed to create trust within the whole collaboration. Funding from venture capitalists and banks belongs to important social capital. Funding from companies is used as an input that can leverage capital to attain more mature projects.
- Pre-production process: After assessment and technological and funding preparation, SIBET initiates the preproduction process, carefully polishing every manufacturing detail and sending collaborating companies detailed instructions. Selected engineers work closely with recruited workers to help them learn how to operate and keep improving the workflow.
- Output: The goal is to successfully incubate companies that can be competitive on technology and price, apply for certifications, and push for aggressive marketing.
To verify the model, SIBET has set a timeline to see if the whole machine runs smoothly. This road map can be divided into three phases.
- Preparatory phase: June-December 2013, build preproduction platform, project pool, and expert pool.
- Trial operation phase: June-December 2014, finish translation of ten projects, incubate five companies, and let two companies that have finished incubations run independently.
- Regular operation phase: January 2015-June 2016, finish translation of 30 projects, incubate 30 companies, and get a state incubator award.
The Journey
SIBET has expanded to about 208 acres with an initial funding of 0.78 billion renminbi (RMB) (~US$127.2 million). SIBET’s research fields now cover medical instrumentation, medical materials, and biological reagents. Current R&D projects include laser medicine, biomedical measurement, medical imaging, biomedical electronics, artificial organs and biomaterials, and biomedical reagents.
So far, SIBET has cofounded the Medical Instruments Industry Alliance of Jiangsu Province, coestablished the Medical Instrument Industry Park, and founded four companies, including ChangGuang Hua Ray Medical Technology (Co. LTD) and Suzhou Keyi-Sky Semiconductor Technologies Inc. Total capital has mounted to 65 million RMB (~US$10.6 million). SIBET is moving forward and gradually realizing its dream.
To accelerate its research progress, SIBET has not only recruited many renowned researchers but also established substantial collaboration with world-famous institutes and universities. For example, Dr. Eliot McVeigh (Johns Hopkins University, Baltimore, Maryland) and Dr. Hongjie Dai (Stanford University, California) have agreed to join our international advisory board. Dr. Xinde Li (Johns Hopkins University), Dr. Dinggang Shen (University of North Carolina, Chapel Hill), and Dr. Huabei Jiang (University of Florida, Gainesville) have also agreed to be on our overseas advisory board. So far, one expert has been awarded a position by the Thousand Talents Program by the Chinese central government and seven experts in different fields have been recruited by the Hundred Talents Program of the CAS. SIBET insists on taking in young and well-qualified talents. More than 75% of the staff are under 30, and 82% have master’s degrees or above.
SIBET is still a young institute struggling to pursue a dream, but the road to that dream seems thickly sown with thorns. SIBET has to guarantee a continuous supply of professional talent and funding support for the operation of the translation platform. So building this talent and funding pool is a top priority. Fortunately, SIBET recently cofounded the Suzhou Share High-Tech Medical Devices Industry Development Investment Fund with several venture capital companies. The fund has amounted to 300 million RMB (~US$48.9 million) and will be invested in the SIBET translation platform for the incubation of promising projects.
Chaim Weizmann, the first president of Israel, once said, “Miracles sometimes occur, but one has to work terribly hard for them.” Through our hard work with the SIBET translational platform, we hope to establish a distinctive and renowned institute in the area of biomedical engineering.
Acknowledgment
This article is supported by Jiangsu Science and Technology Plan Projects Fund (BE2012651).



