Neuroengineering brings tools and techniques from the engineering fields into neuroscience to create new approaches for investigating the central nervous system (CNS). This fusion of disciplines is advancing our knowledge of how the CNS works and how we can enhance our natural cognitive and emotional function and restore neurological functions that are compromised by disease or injury.
For instance, to deal with the extremely large data sets that arise from trillions of interactions among our neurons, neuroengineering often draws on computational and statistical approaches. Techniques from electrical, chemical, and mechanical engineering, as well as from signal processing, are also frequently incorporated into experimental and clinical neuroscience. Such fusion of disciplines has greatly accelerated the development of rehabilitative and therapeutic interventions. This article highlights a few areas where neuroengineering is making unique and valuable contributions.
Neuroprosthetics
One area in which neuroengineering is making significant progress is prosthetics. Advances in design and mechanics have produced state-of-the-art limb prosthetics that realistically mimic physiological movements. Such artificial substitutes are also equipped with kinetic and pressure sensors that give real-time information on whether the prosthesis is opening or closing, the speed of the motion, whether a prosthetic hand has touched an object, and the extent of grip firmness.
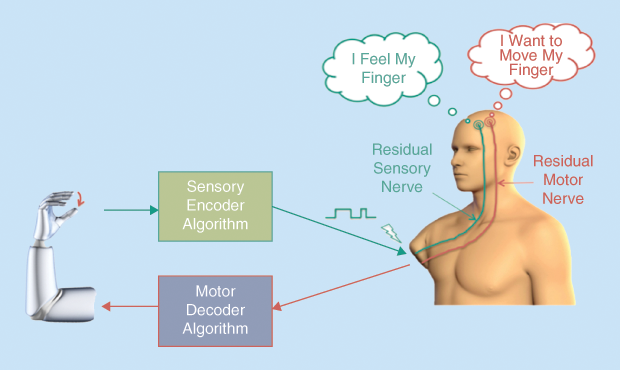
However, this mechanical feedback is not returned through the amputee’s nerves, nor does it reproduce naturally modulated motor control, proprioception, or sensation. Thus, neuroengineering aims to enable prosthetic sensors and motors to communicate, respectively, with the residual sensory and motor nerves in the amputee’s stump, so that the amputee can use natural neural processes to drive the prosthesis. This is expected to make the prosthesis move and feel more like a natural limb.
Many engineering and neuroscience challenges must be addressed to achieve successful communication between the prosthesis and the nervous system. On the engineering side, for instance, the design of implanted electrodes to record nerve signals and communicate them to the prosthesis control unit will be challenging. These electrodes must be small enough to fit around nerves, yet maintain stable contact for stimulation or recording; support the measurement of small-amplitude neural signals; and have durable leads that resist damage during movement.
Transmission between electrodes and the prosthesis control unit should be wireless to minimize connection breakdowns but support fast transmission speeds for real-time performance. The battery must have a large charge capacity for long operation hours and be small and lightweight to maintain prosthesis agility. To fit several in a prosthetic hand, the motors also need to be small and lightweight yet still provide precise movement, accurate performance, superior mechanical operation, ease of maintenance, and durability.
On the neuroscience side, challenges include developing control algorithms that facilitate sensory and motor communications between the prosthesis and nervous system. For instance, for the prosthesis to move proportionally to the amputee’s motor intent, a motor decoder algorithm must decipher the residual motoneurons’ activity, extract the characteristics of the intended movement (open/close, speed, force), and command the prosthesis motors accordingly. Additionally, for the amputee to feel objects as the prosthesis moves, a sensory encoder algorithm needs to integrate the electrical signals from the prosthesis sensors and encrypt this information into a dynamic waveform (of varying amplitude and frequency) that stimulates the amputee’s residual sensory nerves to evoke the natural sensation of touch. Finally, the motor decoder and sensory encoder algorithms must run concurrently and in real time to achieve true closed-loop sensorimotor prosthesis control (Figure 1).
These challenges may be daunting, but the fusion of engineering and neuroscience knowledge into novel neuroengineering approaches has greatly accelerated the advances needed to produce this technology. A prosthesis that goes beyond alleviating motor disability and feels and functions like a natural limb will represent a milestone achievement in the field of neuroengineering.
Neurodegenerative Diseases
The unique contributions of neuroengineering were recently illustrated in the investigation of the neurodegenerative disease amyotrophic lateral sclerosis (ALS). This incurable, fatal disease paralyzes patients and usually results in death within 1–3 years after diagnosis—and the only current treatment extends life by just three months. ALS involves many concurrent pathological changes, and determining primary versus secondary pathologies is difficult because the interactions among these changes are multidirectional.
One challenge with ALS—and with neurodegenerative disease in general—is that, by the time symptoms emerge, a large proportion of neurons (~70% in ALS [1]) have already degenerated. This greatly diminishes the leverage and so the success of treatment efforts. Neurodegenerative symptoms are often delayed because disease changes are usually countered by compensatory changes as neurons struggle to maintain normal function. This dynamic interaction of changes has been observed in spinal motoneurons in the presymptomatic stage of ALS: several concurrent cellular changes occur that have opposing effects on the motoneuron’s overall net excitability. These include changes in cell size, biophysical properties of the membrane, and sodium and calcium ion currents, but the motoneurons display normal net excitability [2], [3]. This pseudonormal net excitability masks the dynamic interactions between disease changes and compensatory changes, while the motoneurons continue to degenerate.
Our own group’s neuroengineering investigation has focused on examining the separate effects of all observable cellular abnormalities on neuronal excitability and on revealing additional abnormalities that might not have been identified via experimental means. Experimental approaches can answer many questions through cycles of trials, but the nonlinear interactions among the components of neuronal excitability are very complex. In addition, many interactions within this system cannot be directly observed or measured by experimental means; thus, we turned to computational simulation in our investigation.
We developed highly accurate neural models to investigate ALS disease changes [3] and specifically used a transformation algorithm to reveal disease changes that are “hidden” from experimental observation. The algorithm begins with a model of the normal cell and then computationally applies each known disease change, one at a time, to show how that change impacts the cell’s excitability. By examining how every change and combination of changes impacted the cell model, we were able to identify additional cellular changes that had not been detected in experiments. This allowed us to reverse-engineer those disease mechanisms that were most likely occurring and thus develop new experiments to verify the impact of these “hidden” mechanisms.
Without the guidance of the computer simulation, we would not have been able to reveal these masked abnormalities. However, although simulations can greatly accelerate the process of discovery, experimental confirmation is critical to determine the simulation predictions that are actually correct. Therefore, a fused neuroengineering approach, with computer simulations predicting cellular abnormalities and experiments verifying model predictions, could be very efficient in identifying novel potential therapeutic targets for ALS.
Brain-Machine Interface
Neuroengineering also aims at recording neural intentions directly from the brain to move prosthetics, communicate, or control machinery such as wheelchairs or external devices. Such technologies, known as brain–machine interfaces (BMIs), provide an alternative for patients when neural signals from peripheral nerves are not viable, such as in spinal cord injury or neurodegenerative diseases.
BMIs have immense potential to restore a significant degree of independence to people with disabilities: noninvasive electroencephalogram (EEG) measurements have been successfully used to enable paralyzed patients to use eye movements, internally visualized body movements, and auditory stimuli to control computers. For instance, BMIs have allowed paralyzed patients to control computerized communication devices, grasp objects, and, more recently, produce four distinct command options to navigate a virtual car on a twisting course. Such command options could also direct other applications, such as wheelchairs.
However, an EEG conveys limited information: the desired signal is filtered through scalp and bone and is subject to noise from other cortical sites and facial electromyogram signals. Thus, neuroengineering is exploring more focal neural signal detectors.
Subdural electrode recordings, called electrocorticograms (ECoGs), sample a smaller area of cortical activity, thus reducing noise with only a modest increase in risk. Intracranial electrodes, by contrast, offer the potential for recording a broader band of nuanced behavioral intentions, but serious risks must be addressed before translation to clinical use. Successful animal experiments using intracranial implants have allowed rats and rhesus monkeys to move simple robotic devices with reaching and grasping motions. Notably, some rats and primates initially used movements to control the device and then adapted to using their brain activity (intention to move) without performing the movement. More recently, intracranial implants allowed human patients to perform force-modulated grasping tasks with a robotic arm.
Although these examples are promising, testing in human patients must be a slow, cautious process. There are risks of infection when intracranial electrodes connect to hardware outside the skin, although wireless probes are being explored to eliminate this issue. In addition, implantation can damage brain tissue, and probes can cause inflammatory reactions. Furthermore, whereas implants must function for years, current probes have a use span of months, after which they typically become overgrown by fibrous tissue and local brain cells begin to die. Biochemical approaches have been suggested, such as coating the probe with anti-inflammatory agents to reduce fiber overgrowth and with growth factors to counter cell death. Technology barriers also exist: BMIs, like prosthetic limbs, need near-real-time control algorithms.
In sum, effective intracranial BMI devices are not likely to be translated to human patients as quickly as less-risky EEG and ECoG technologies. However, constant advances are being made through neuroengineering approaches, and BMI technologies hold great potential for improving the lives of patients with neurodegenerative diseases, spinal injury, cerebral palsy, and other similar conditions.
Interacting with Machines: Exchanging Thoughts and Emotions
As neuroengineering accelerates BMI development, futuristic technologies could become a reality over the next few decades. Advances in artificial intelligence (AI) are making true intellectual human–machine interactions a possibility. Researchers seek to incorporate the differing strengths of human and AI intelligences to increase the capabilities of human–machine collaborations. This requires improving AI’s understanding of human language and gestures, enabling robots to respond to commands more quickly and accurately. Neuroengineering is also advancing robotic problem-solving algorithms to enable more independent task performance. A robot with natural language capabilities and basic problem-solving functionality could perform many tasks of daily living for disabled patients.
Another type of human/AI interface now being tested is the decision-making support platform. While humans show more creativity in formulating options and predicting potential outcomes, AI systems have far greater ability to manage vast amounts of data and calculations. Thus, for example, in combat situations, AI could condense incoming reports and track resources, thus freeing military commanders to better assess the overall situation and make more fully informed decisions. Because emotions are involved in human decision making, neuroengineering researchers are also exploring the development of empathetic capabilities in AI systems. Again using the military example, an AI assistant could employ optical or auditory scanning algorithms to detect increased stress in a commander’s microexpressions or voice. This could trigger a higher level of independent operation on the part of the AI to reduce the minor decisions the commander handles. Efforts are already underway to build a reliable emotion-recognition algorithm through the compilation of a vast database of facial expressions captured in response to known content. In addition, ongoing work is advancing the identification of predictive links between speech patterns (word choices, pauses, tone) and emotions to create a speech-based emotion-recognition algorithm.
Current BMI platforms are also being developed to interpret brain signals involving thought–emotion interactions to advance our understanding of mood disorders. Such an application could provide biofeedback to patients who are learning cognitive behavioral techniques to modulate their mood. An even more futuristic application has also been envisioned: a BMI platform that detects dysfunctional brain activity and then delivers therapeutic stimulation. To achieve such an advanced platform, we must advance the decoding of neural patterns to accurately categorize normal from dysfunctional activity.
Although many barriers remain before we can treat or cure complex diseases such as ALS, replace lost limbs with prostheses that feel and move naturally, control machines with our minds, or develop true human–AI partnerships, progress is constantly accelerating. We expect that the neuroengineering field will continue to recruit an ever-widening range of disciplines and remain at the forefront in advancing these critical efforts to improve the lives of people globally.
Acknowledgments
Neuroengineering research is supported/sponsored by the following organizations: the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office Hand Proprioception and Touch Interfaces (HAPTIX) program, under the auspices of Dr. Doug Weber through the DARPA Contracts Management Office (contract no. HR0011-15-C-0083); the National Institute of Neurological Disorders and Stroke (grant no. NS091836); and the Air Force Research Laboratories (grant no. 670396).
References
- J. Hegedus, C. T. Putman, and T. Gordon, “Time course of preferential motor unit loss in the SOD1G93A mouse model of amyotrophic lateral sclerosis,” Neurobiol. Disease, vol. 28, no. 2, pp. 154–164, 2007.
- K. A. Quinlan, J. E. Schuster, R. Fu, T. Siddique, and C. J. Heckman, “Altered postnatal maturation of electrical properties in spinal motoneurons in an ALS mouse model,” J. Physiol., vol. 589, no. 9, pp. 2245–2260, Feb. 2011.
- S. M. Elbasiouny, J. Amendola, J. Durand, and C. J. Heckman, “Evidence from computer simulations for alterations in the membrane biophysical properties and dendritic processing of synaptic inputs in mutant superoxide dismutase-1 motoneurons,” J. Neurosci., vol. 30, no. 16, pp. 5544–5558, Apr. 2010.