“Did you get it all?” That’s one of the first questions many cancer patients ask when they awake following surgery to remove a tumor. Surgeons today can only say they did their best and resected as much as they could given the current methods. A number of new dyes, however, are beginning to offer better options for distinguishing tumor from normal tissue, and may one day allow surgeons to tell patients that, yes, they did indeed remove it all.
These dyes are molecules that latch only onto tumor cells, fluoresce, and show surgeons the entire tumor, including any of its elusive and meandering finger-like projections that may otherwise escape resection.
Some of the new dyes include 5-aminolevulinic acid, or 5-ALA, which is already being used for guidance of brain-tumor surgery in Europe; a “tumor paint” that is based on a peptide found in scorpion venom; and a fluorescently labeled molecule to light up a wide variety of tumors.
5-ALA Crossing Barriers

Marketed as Gliolan in Europe, 5-ALA received approval there in 2007, and has also been used in many other countries around the world to assist in surgeries to remove adult malignant brain tumors, or gliomas. Not yet approved in the United States, it is now being submitted to the U.S. Food and Drug Administration for fast-track approval, says neurosurgical oncologist Constantinos Hadjipanayis, M.D., Ph.D., who has been trying to bring 5-ALA to America for more than six years (1). Hadjipanayis is professor and chair of the Mount Sinai Beth Israel Department of Neurosurgery and director of neurosurgical oncology for the Mount Sinai Health System, and he also directs the Mount Sinai Brain Tumor Nanotechnology Laboratory in New York (Figure 1, right).
Ever since Hadjipanayis first saw 5-ALA lighting up tumor cells in vibrant red, he was hooked. He sought FDA authorization to use 5-ALA, which is classified as an investigational new drug, and began a study of his own at Emory University in Atlanta, Georgia, in 2011, and is continuing that work today at Mount Sinai as sponsor of a multicenter trial.
5-ALA is an orally administered compound that makes its way into the blood, crosses the blood-brain barrier, and selectively accumulates in brain tumor cells within about three hours. Once there, it exploits metabolic differences in the tumor cells and gives off a fluorescent byproduct. Using an operating microscope outfitted with optic filters, the surgeon employs a specific wavelength of light to see the tumor’s violet-red glow against a backdrop of bluish-hued normal tissue (Figure 2).
“I’ve used it in a number of patients in Atlanta and in New York, and this agent makes the tumor obvious,” Hadjipanayis says. The ability to detect tumor tissue by fluorescence is unprecedented. A number of studies have confirmed that the use of 5-ALA also achieves a significantly higher rate of more complete resections of gliomas as compared to conventional methods. “Beyond that, we can see the tumor and understand its relationship to other tracks in the brain, which can help us perform the surgery more safely,” he notes.
Another benefit of 5-ALA is its ability to show the surgeon the tumor in real time, Hadjipanayis explains. In conventional surgery, a pre-surgical magnetic resonance imaging (MRI) picture of the patient’s brain is loaded into a neuronavigation machine, which guides the surgeon during the operation. “Unfortunately, the brain can shift from the time the MRI was taken to the time of surgery, and that can throw you off,” he says. 5-ALA’s real-time imaging capability eliminates that problem.
He believes the FDA appreciates the value of 5-ALA for its safety and for visualizing tumor tissue that can result in more complete tumor resections, which can potentially reduce the need for follow-up surgeries, and will hopefully grant approval in 2017. “Then all neurosurgeons will be able to use it,” Hadjipanayis notes.
Venom-related Dye

Another dye that is in the works is a “tumor paint” based on chlorotoxin, which is one of the many constituents of scorpion venom. Chlorotoxin binds to glioma cells without harming brain tissue. About 10 years ago, neuro-oncologist James Olson, M.D., Ph.D., and his lab at the Fred Hutchinson Cancer Research Center in Seattle began working with other researchers at Seattle Children’s Hospital and the University of Washington to combine synthesized chlorotoxin with a fluorescent dye as a means to pinpoint tumors (2).
“Dr. Olson put in a lot of effort doing the basic biology characterization of the compound, which is a peptide, and saw its clinical potential. That’s when he began working with Heather Franklin to found a company, Blaze Bioscience, around this technology,” says Dennis Miller, Ph.D., senior vice president of development of the Seattle company, which was established in 2011 (Figure 3, right). The company named the fluorescent chlorotoxin BLZ-100.
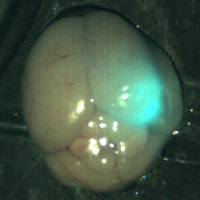
Unlike 5-ALA, BLZ-100 is injected into the patient prior to surgery. The compound circulates through the blood and likely binds to one of the heterotetramers on the cholesterol-rich lipid rafts that are present on most tumor cells. Once BLZ-100 binds and is internalized in the tumor cell, the surgeon is then able to shine a near-infrared device on the surgical field and spot the glowing tumor and its margins (Figure 4, right: Through the use of tumor paint BLZ-100 and visualization with near-infrared light, a tumor in a mouse brain glows brightly. (The smaller white areas are simply light reflection.) Image courtesy of Blaze Bioscience, Inc.).
Although BLZ-100 has the potential to work on a wide range of cancers, the company decided to focus on brain cancer and pediatric neurological cancer, two areas where surgical resection is the best curative option. BLZ-100 is currently undergoing phase I clinical trials. As of early November 2016, surgeons had used BLZ-100 on more than 70 patients, including about a dozen pediatric patients, according to Miller. “So far, the safety profile is very encouraging; there’s very little toxicity associated with this agent, and we’re seeing a large number of cases where the tumor is taking up the dye, so it’s capable of showing us the fluorescence that we want,” he says (3). If all goes well, Miller hopes it will make it to market in 2019, possibly earlier.
As that work proceeds, Blaze Bioscience is developing specialized near-infrared imaging devices that integrate well with the equipment surgeons already use in the operating room, Miller adds. At the same time, the company is beginning to look beyond brain and pediatric cancer, and studying BLZ-100’s applicability to breast cancer.
“Within the realm of cancer care, if surgery is not successful, that’s when patients get into these loops of having to go back for more surgery or go back for more intensive chemotherapy,” he remarks. “I’m hopeful that our work is going to put more patients on the right first step so they will really have the best chance of being cured.”
Color Coding the Bad and Good
A lab at the University of California San Diego (UCSD) is tackling cancer surgery on two fronts: It is developing an injectable dye to make a wide variety of different tumors glow, while also developing a second dye to light up nerves. This would allow surgeons to skirt the nerves while resecting tumor tissue, a capability that is critical in prostate cancer, cancers of the head and neck, and other surgeries where patients are often as concerned about removing the tumor as they are about preserving full function.
For the tumor dye, the UCSD research group of Quyen Nguyen, M.D., Ph.D., developed a cell-penetrating peptide that takes advantage of a cancerous tumor’s use of enzymes, specifically proteases, to destroy normal tissue and make a space for itself. “Our thought was to leverage that enzymatic activity and make a molecule that basically turns a different color when it’s chewed up by these enzymes that tumors make,” explains Nguyen, who is a surgeon and an associate professor in the UCSD Department of Surgery (Figure 5). The intact molecule comprises two dyes: the presence of one prevents the second one from glowing. “When the enzyme of the tumor attacks the molecule, however, the two dyes separate, you can see a color change from bluish to red. The red tells you to cut here,” she describes, noting that the surgeon need only use one of several readily available advanced cameras to see the color shift.

Avelas Biosciences of La Jolla, California, licensed Nguyen’s molecule from UCSD, has just completed phase I human breast cancer trials, and as of November 2016 was gearing up to submit the data to the FDA to begin phase II trials, she reports.
As that progresses, Nguyen and her research groups are working on second-generation, ultra-specific molecules designed to deliver radiosensitizers that would make tumor cells more susceptible to radiation. “That way, you would be able to deliver less radiation and get less adjacent tissue toxicity, and yet have the same or better efficacy,” she explains.
At the same time, they are also pursuing similar molecules to light up nerves (5, 6). For this technology, they are using different fluorescently labeled peptides to bind to the nerves, Nguyen says. Such a nerve-finding molecule could also potentially carry compounds to alleviate cancer pain or other nerve-induced conditions, such as renal-vascular hypertension or hyperhidrosis, she contends.
DYE, DYE, DYE!
Nguyen, Olson, and Hadjipanayis are taking different approaches, but all agree that dyes will go a long way toward helping surgeons resect tumors more completely.
Nguyen remarks, “I think that everyone is now recognizing that there is an opportunity to introduce molecular imaging or fluorescence to enable surgeons in the operating room to see the difference between cancer and normal far better than what we’ve been able to see with white light only.”
Adds Hadjipanayis, “The goal is for patients to live longer. That’s what we want.”
References
- Hadjipanayis C. G., Widhalm G., and Stummer W. “What is the surgical benefit of utilizing 5-aminolevulinic acid for fluorescence-guided surgery of malignant gliomas?” Neurosurgery, vol. 77, no. 5, pp. 663-73, November 2015.
- Veiseh, V., Gabikian, P., Bahrami, S-B., Veiseh, O., Zhang, M. Hackman, R. C., Ravanpay, A. C., Stroud, M. R., Kusuma,Y., Hansen, S. J., Kwok, D., Munoz, N. M., Sze, R. W., Grady, W. M., Greenberg, N. M., Ellenbogen, R. G., and Olson, J. M. “Tumor paint: A chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci,” Cancer Research, vol. 67, no. 14, pp. 6882-6888, 15 July 2007.
- Baik, F. M., Hansen, S., Knoblaugh, S. E., Sahetya, D., Mitchell, R. M., Xu, C., Olson, J. M., Parrish-Novak, J., and Méndez, E. “Fluorescence identification of head and neck squamous cell carcinoma and high-risk oral dysplasia with BLZ-100, a chlorotoxin-indocyanine green conjugate,” JAMA Otolaryngology – Head & Neck Surgery, vol. 142, no. 4, pp. 330-338, April 2016.
- Metildi C. A., Felsen, C. N., Savariar, E. N., Nguyen, Q. T., Kaushal, S., Hoffman, R. M., Tsien, R. Y., and Bouvet, M. “Ratiometric activatable cell-penetrating peptides label pancreatic cancer, enabling fluorescence-guided surgery, which reduces metastases and recurrence in orthotopic mouse models.” Annals of Surgical Oncology, vol. 22, no. 6, pp. 2082-7, June 2015.
- Hussain, T., Mastrodimos, M. B., Raju, S. C., Glasgow, H. L., Whitney, M., Friedman, B., Moore, J. D., Kleinfeld, D., Steinbach, P., Messer, K., Pu, M., Tsien, R. Y., Nguyen, Q. T. “Fluorescently labeled peptide increases identification of degenerated facial nerve branches during surgery and improves functional outcome,” PLoS One, vol. 10, no. 3:e0119600, 9 March 2015.
- Whitney, M.A., Crisp, J. L., Nguyen, L. T., Friedman, B., Gross, L. A., Steinbach, P., Tsien, R. Y., and Nguyen, Q. T. “Fluorescent peptides highlight peripheral nerves during surgery in mice,” Nature Biotechnology, vol. 29, no. 4, pp. 352-356, April 2011.