Simulation is any artificial construct that represents a real-world process. Technology-enhanced simulation in modern health care is growing exponentially, but the basic concept of “practice” to enhance real-world performance in medicine dates back centuries. For example, in the 1600–1700s, birthing simulators were built from human bones, leather, wood, and wicker to train midwives how to manage common birthing emergencies [1].
Today, simulation sessions can be loosely categorized into three formats: 1) imaginative scenario-based work (paper or tabletop simulations), 2) individual versus team-based manikin simulations or standardized patient-based simulations, and 3) specific task-based trainers. Modern surgical simulation focuses largely on the latter two approaches and is typically differentiated from other forms of medical simulation by the use of specific surgical models or tasks during the simulation. However, despite the rapid growth of simulation in health care, operative training models that replicate the practice of surgery remain underdeveloped.
Currently, there is a robust discussion in the simulation community regarding center-based versus in situ simulation locations. Center-based simulation typically occurs outside of a patient care facility. These centers are flexibly designed to accommodate a wide variety of participants from all health care groups, as well as different patient care settings [i.e., inpatient wards, outpatient clinics, emergency department bays, radiology imaging suites, or operating rooms (ORs)]. While such flexibility allows for a wide range of participants and settings, it often limits exact replication of any one particular environment’s physical space and equipment.
In situ simulation is defined as simulation in the learners’ actual work environment. Advantages of in situ locations include lower equipment and overhead costs, ability to train more participants in a given amount of time, enhanced simulation realism, identification of latent system errors, and improved convenience for both the learners and faculty [2]. However, in situ locations generate unique logistical and organizational challenges. In this article, we describe our experience in integrating simulation into the existing OR complex at Massachusetts General Hospital (MGH). We hope this description fosters increased medical collaboration with engineering communities, not only with respect to the advancement of realistic surgical modeling, but also in designing integrated systems to serve both clinical and educational needs in the health care environment.
Surgical History of MGH
The MGH in Boston currently performs over 37,000 operations a year where more than 1,000 medical students, residents, and fellows learn. Inside the MGH Bulfinch Building is the Ether Dome, which is the site of the first public demonstration of ether anesthetic for a surgical operation in the United States. On 16 October 1846, William T.G. Morton, a Boston dentist, successfully administered ether to Gilbert Abbott, which allowed MGH cofounder, Dr. John Collins Warren, to painlessly remove a tumor from Mr. Abbott’s jaw.
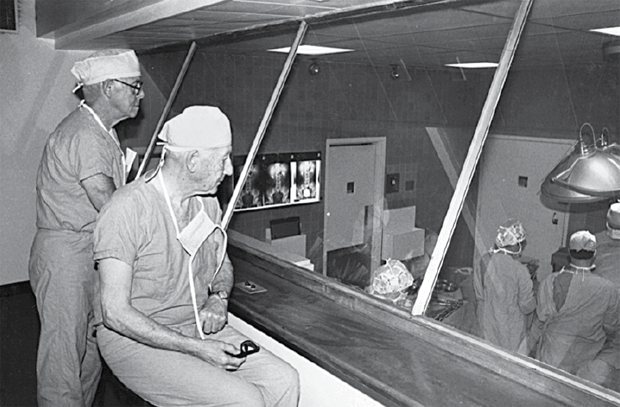
This landmark event at MGH started a long tradition of performing operations that select members of the public and medical community could observe from a platform surrounding the OR table. This open environment ceased with increased understanding of germ theory and the importance of sterility to prevent surgical infection. However, in 1939, MGH designed enclosed surgical observation decks for the opening of the ORs in the White Building. These former “state-of-the-art” two-story, sealed observation decks overlooked several OR theaters (see Figure 1). Except for the one overlooking OR#5, these historical decks were ultimately closed, and most were demolished. In 2012, a wing of new ORs opened in the MGH Lunder Building, allowing three of the original White Building ORs, including OR#5 and its observation deck, to be used for simulation training.
A New Vision for OR Training
The vision for the new in situ OR simulation suites was to provide a physical space to conduct high-fidelity OR team training simulations; to allow practice opportunities for individual operative skills; and to test new OR devices, procedures, and policies. This vision was integrated with overall institutional goals of discovering latent organizational risks, supporting residency training and nursing education programs, and promoting the continued professional development of health care providers through high-quality educational offerings. We also recognized that the ultrahigh-fidelity nature of this simulation space would be an ideal laboratory to develop and test new assessments of individual and teamwork performance within an OR environment.
The concept of creating simulation training opportunities within an active OR complex presented exciting opportunities as well as important challenges. In addition to its inherent authenticity, the idea of an in situ surgical simulation space had definite historical appeal, harkening back to MGH’s use of observation galleries as an educational tool. Financially, there were also real cost savings to using existing facilities rather than building a brand new surgical simulation center. Also, OR staff participating in simulation sessions saved considerable time by eliminating travel to an off-site training locale. The participants could then return promptly to their clinical responsibilities, thereby increasing overall employee productivity for their departments.
However, utilization of the existing physical OR space did not allow for renovations typical of an off-site simulation center (removing/moving walls, adding one-way mirrors, etc.). In addition, management of the operational workflow of an in situ simulation center internal to a live operating suite was critical. Some of the critical questions considered included the following: Would all equipment used in the simulation center be part of the active OR inventory? Would equipment ever be used that was not part of the active OR inventory? How would sterile supplies be maintained? Would we use real or simulated medication vials? If using real medication vials, how would we balance this against the ongoing national medication shortages? How would actual patients presenting for surgery and passing through the hallway adjacent to a simulation perceive their experience? What would happen if a simulation participant left the simulation environment to obtain help from nonsimulation participants?
These questions helped us develop guiding principles and policies to ensure that the in situ simulation supplies, equipment, and processes were “sealed” and that no training equipment or processes “leaked” into the real ORs. To this end, we created OR simulation policies, a standardized orientation for all simulation participants, reviewed simulation safety protocols at the beginning of each session, installed explicit signage for all simulation spaces, and attached “simulation-only” labels for all supplies and equipment. As an additional fail-safe to avoid cross-contamination of any “mock” elements into the real-world environment, we stocked and maintained only clinical-grade equipment, supplies, instrumentation, and medications in each simulation room. We worked closely with our perioperative biomedical engineering and pharmacy groups to ensure that these elements are maintained regularly and match the real ORs. We also carefully designed several engineering work-arounds of the existing phone/overhead intercom, anesthesia machine, manikin, clinical monitoring, and computer systems, so that our training activity would not interfere with routine OR operations. The observation deck acts as a control area to operate the manikin and other simulation elements; it also provides an opportunity for individuals to view the proceedings, out of sight of the simulation participants.
Similarly, we worked closely with our perioperative information technology group to create patient names and medical record numbers that would only be used for the patients in the simulation scenarios. This opened up the potential capability to track supplies, equipment, and medication costs for the simulated patients, just as we would in a real case. We also worked with the MGH Laboratory for Computer Science to create a radio-frequency identification badge reader system that allowed simulation participants to sign in for each simulation session with a simple badge swipe. This system allowed us to easily see who was assigned to take care of a simulated patient for that session documented in our OR computer systems. The simulated patient also appeared on the real OR schedule so that the OR community could see when the rooms were being used and what “operation” the teams were going to perform. These entries served to highlight the concept of continuous education as a key element of daily operations and emphasize the connection between simulation and actual patient care.
Training Experience and Future Needs
We started using our new in situ simulation ORs for operative training in late 2012. Since that time we have conducted nearly 400 OR simulations for many specialties, including general surgery, pediatric surgery, burn surgery, laryngology, oral maxillofacial surgery, urology, obstetrics and gynecology, orthopedics, thoracic, and cardiac surgery. Our multidisciplinary approach has included surgeons (attending/resident physicians), anesthesia providers (attending/resident physicians and certified registered nurse anesthetists), registered nurses, and surgical technologists, which helps to enhance and enrich the educational opportunities. Over 1,000 health care staff have participated in OR simulation experiences as a partial fulfillment of various curricula and continuing education initiatives including medical malpractice discounts, continuing education credits, Accreditation Council for Graduate Medical Education milestone achievement, Anesthesia Crisis Resource Management curriculum, and the American College of Surgeons and the Association for Program Directors in Surgery National Surgical Skills Curriculum.
While the cognitive and teamwork experiences in our simulations are quite realistic, there remain technical gaps that present challenges unique to the in situ simulation environment. Only close collaboration with inventors and engineers will yield high-fidelity surgical models that can be fully integrated into an already complex operative environment. For example, our manikins were not originally designed for regular positive pressure ventilation from an anesthesia machine, so several modifications were made to ensure adequate pulmonary compliance to avoid false alarms from the anesthesia machine for low tidal volumes/pressures and air leaks. The manikins were also modified to prevent internal damage from irrigation fluids, sharp cutting instruments, or simulated blood. Various surgical models, such as an abdomen for laparoscopic operations and a bleeding tumor, had to be engineered and placed on top of the manikins due to lack of internal space.
New modifications are continually needed to create OR situations that are realistic enough to engage the surgical team (surgeons, scrub technologists, circulator nurses, and anesthesiologists) without being excessively bulky or damaging to the manikin. In particular, computerized algorithms that could mimic ventilator characteristics and response to medications would remove the need for pumps and bellows within the manikin’s thoracic and abdominal cavities. The removal of this equipment would enhance internal space within the manikin and allow direct internal integration of surgical models to enhance the scenario realism and the overall engagement of surgical teams.
In the future we hope to make simulation staffing and resources even more accessible to surgical teams as part of their routine OR workday. Previous research on the effectiveness of dress rehearsals prior to actual surgery has been shown to benefit teams who routinely practice with simulation [3]. We also feel that in situ simulation allows teams to practice in their own environment as opposed to traveling to a simulation center. This benefit appears to enhance accessibility and overall learner satisfaction with the simulation sessions.
In summary, the development and integration of three in situ simulation ORs into the working OR clinical environment has required advanced vision, creative use of OR space, and ongoing collaboration across hospital services. Beyond their profound educational value, these rooms have provided robust opportunities to refine OR policies and procedures, enhance the OR safety culture, and support collaborative research opportunities, all of which help us to continuously improve patient care. We are currently exploring the development of simulation-based credentialing metrics that could be incorporated in the Joint Commission processes. We look forward to expanding our work with engineering communities to help advance the field of surgical simulation modeling and operations.
References
- A. A. Wilson, “New synthesis: William Smellie,” in The Making of Man-Midwifery: Childbirth in England 1660–1770. London: Univ. College London Press, 1995.
- K. M. Ventre, J. S. Barry, D. Davis, V. L. Baiamonte, A. C. Wentworth, M. Pietras, L. Coughlin, and G. Barley, “Using in situ simulation to evaluate operational readiness of a children’s hospital-based obstetrics unit,” Simul Healthc. vol. 9, no. 2, pp. 102–11, Apr. 2014.
- J. D. O’Leary, O. O’Sullivan, P. Barach, and G. D. Shorten, “Improving clinical performance using rehearsal or warm-up: an advanced literature review of randomized and observational studies,” Acad Med., vol. 89, no. 10, pp. 1416–22, Oct. 2014.



