Cancer represents a compilation of diseases characterized by rapidly dividing, invasive cells. Worldwide data indicate that over 14 million new cancers were diagnosed in 2012, with a projected increase of more than 19 million diagnosed cases by 2025 [1]. Survival rates for some cancers have increased dramatically, but there are still cancer types for which the prognosis is poor and few treatments exist. Thus, there is a growing need for new therapies targeting these difficult-to-treat cancers.
TTFields: A New Treatment Modality
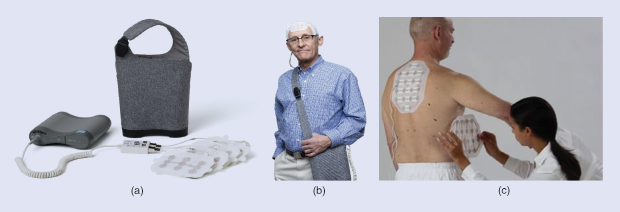
The three traditional modalities for treating cancer are surgery, radiation, and systemic therapies including chemotherapy, hormonal therapy, and immunotherapy. Addressing the need for novel treatments for difficult-to-treat cancers, a new regional modality termed tumor treating fields (TTFields) has emerged, which is proving its place in the anticancer armamentarium. TTFields are low-intensity (1–5V peak to peak) alternating electric fields in the frequency range of 100–500 kHz that interfere with cell division in rapidly replicating cancer cells. This breakthrough technology is noninvasive, as treatment is delivered through transducer arrays placed on the patient’s body (Figure 1). The main adverse effect associated with TTFields is skin irritations that occur in regions where the arrays are placed on the body. The U.S. Food and Drug Administration (FDA) has approved TTFields for the treatment of newly diagnosed and recurrent glioblastoma multiforme (GBM), an aggressive primary brain tumor.
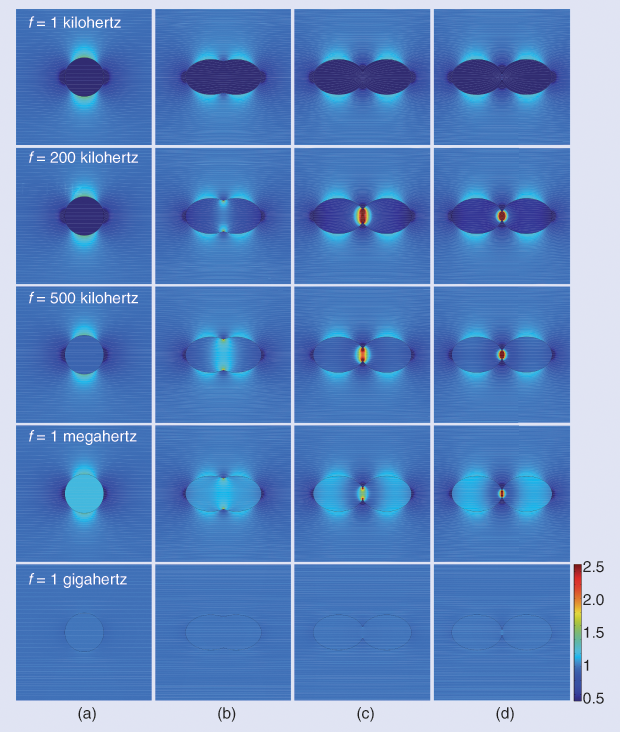
The development of TTFields began in 1999, when Prof. Yoram Palti of the Technion Israel Institute of Technology hypothesized that electric fields in the frequency range of 100–500 kHz could inhibit cell division, with potential application in the treatment of cancer. The rationale underlying this hypothesis was that at low frequencies (< 1 kHz) the dielectric cell membrane effectively shields the cytoplasm from external fields, and at high frequencies (> 1 MHz) the membrane and cell become essentially transparent to the electric field (Figure 2). Therefore, electric fields within the frequency range of 100–500 kHz could potentially penetrate dividing cells and disrupt essential processes and cellular structures, leading to cell death. Prof. Palti then demonstrated that electric fields in the frequency range of 100–300 kHz disrupted the mitotic spindles of rapidly dividing cancer cells, thus killing them.
Treatment Developments Using TTFields
Over the last two decades, interest in TTFields has increased, and the number of research groups investigating the fields has grown steadily, establishing them as an efficacious anticancer modality. A pilot study begun in 2004 validated the feasibility of treating GBM with TTFields [2]. This study led to a phase III clinical trial (EF-11) proving the efficacy and safety of TTFields for recurrent GBM, resulting in FDA approval in 2011 [3].
A second phase III clinical trial (EF-14) of TTFields in combination with the chemotherapy agent temozolomide (TMZ), for newly diagnosed GBM demonstrated that adding TTFields to maintenance TMZ chemotherapy significantly prolonged progression-free and overall survival in newly diagnosed GBM patients [4]. These data led to the 2015 FDA approval of TTFields combined with TMZ for the treatment of newly diagnosed GBM patients. TTFields are currently being tested on other aggressive tumor types in clinical trials related to brain metastases and lung, pancreatic, mesothelioma, and ovarian cancers.
Simulation and Modeling to Optimize Treatment
Optimizing treatment for patients is challenging, and preclinical studies show that the effect of TTFields increases with intensity, which underscores the critical need to understand how TTFields intensities distribute within the body [5]. Because there are no practical means for measuring field intensities within the bodies of patients undergoing treatment, simulations and modeling are the primary tools for obtaining these essential data.
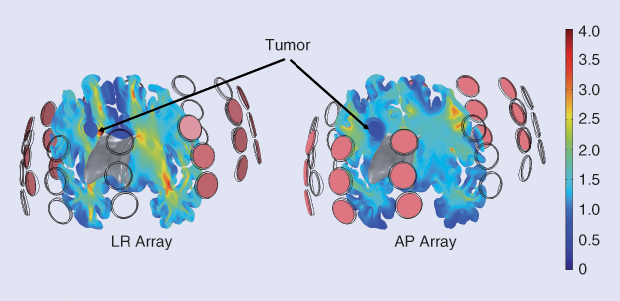
Work on modeling the distribution of TTFields intensities within the body is increasing rapidly and becoming a valuable tool to improve the clinical application of TTFields for cancer treatment. Simulation-based studies using realistic head models have shown that TTFields effectively penetrate brain tissues and that the field distribution is heterogeneous (Figure 3) and highly dependent on individual anatomy [6]. Further, these studies have shown that the distribution of TTFields intensities depends on the location of the arrays on the scalp and that the array position can be variously optimized to deliver maximal field intensities to the tumor. Modeling studies have shown that the selective removal of brain tissue can create low-resistance pathways for TTFields into the tumor tissue; these pathways can lead to an increase of over 60% in field intensities in the gross tumor volume and in the peritumoral zone, leaving the field intensity in the healthy tissue largely unaffected [7].
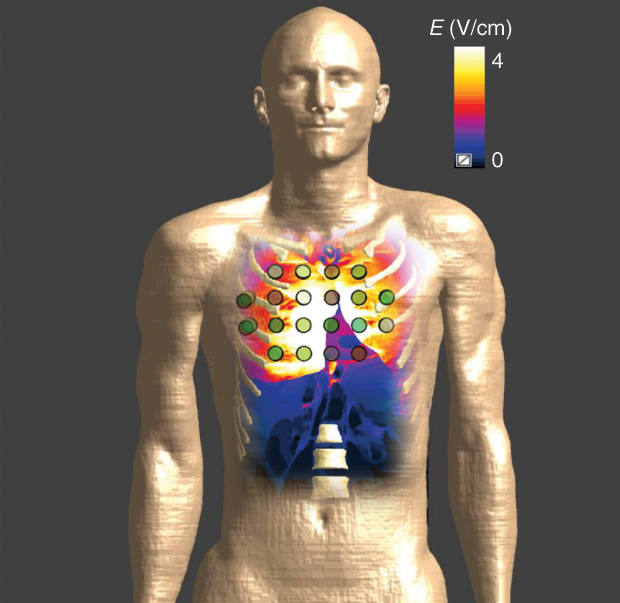
Simulations have also proven that TTFields can be delivered to the thorax at intensities high enough to generate a therapeutic effect (Figure 4). These simulations have shown that, as with brain tissue, the intensity distribution within the thorax depends on where the arrays are placed on the body, providing opportunities to optimize array layout and so maximize the therapeutic effects for the individual patient. Simulations are also being used to evaluate designs of new arrays for the delivery of TTFields to the thorax. These new arrays are designed to adhere well to body contours, while maximizing field intensities delivered to the tumor bed, potentially leading to improved efficacy and increasing patient comfort during treatment.
Improvement of TTFields technology depends on developing efficient modeling tools to rapidly simulate multiple treatment scenarios related to optimal TTFields delivery. These tools increase the understanding of how TTFields distribute within the body, optimizing TTFields delivery and improving patients’ cancer outcomes.
References
- International Agency for Research on Cancer. (2013, Dec. 12). Latest world cancer statistics global cancer burden rises to 14.1 million new cases in 2012: Marked increase in breast cancers must be addressed. World Health Organization. [Online].
- E. D. Kirson, V. Dbalý, F. Tovarys, J. Vymazal, J. F. Soustiel, A. Itzhaki, D. Mordechovich, S. Steinberg-Shapira, Z. Gurvich, R. Schneiderman, Y. Wasserman, M. Salzberg, B. Ryffel, D. Goldsher, E. Dekel, and Y. Palti, “Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors,” Proc. Natl. Acad. Sci. USA, vol. 104, no. 24, pp. 10,152– 10,157, 2007.
- R. Stupp, E. T. Wong, A. A. Kanner, D. Steinberg, H. Engelhard, V. Heidecke, E. D. Kirson, S. Taillibert, F. Liebermann, V. Dbalý, Z. Ram, J. L. Villano, N. Rainov, U. Weinberg, D. Schiff, L. Kunschner, J. Raizer, J. Honnorat, A. Sloan, M. Malkin, J. C. Landolfi, F. Payer, M. Mehdorn, R. J. Weil, S. C. Pannullo, M. Westphal, M. Smrcka, L. Chin, H. Kostron, S. Hofer, J. Bruce, R. Cosgrove, N. Paleologous, Y. Palti, and P. H. Gutin, “NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality,” Euro. J. Cancer, vol. 48, no. 14, pp. 2192–2202, Sept. 2012.
- R. Stupp, S. Taillibert, A. A. Kanner, S. Kesari, D. M. Steinberg, S. A. Toms, L. P. Taylor, F. Lieberman, A. Silvani, K. L. Fink, G. H. Barnett, J. J. Zhu, J. W. Henson, H. H. Engelhard, T. C. Chen, D. D. Tran, J. Sroubek, N. D. Tran, A. F. Hottinger, J. Landolfi, R. Desai, M. Caroli, Y. Kew, J. Honnorat, A. Idbaih, E. D. Kirson, U. Weinberg, Y. Palti, M. E. Hegi, and Z. Ram, “Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical trial,” J. Amer. Med. Assoc., vol. 314, no. 23, pp. 2535–2543, Dec. 15, 2015.
- E. D. Kirson, Z. Gurvich, R. Schneiderman, E. Dekel, A. Itzhaki, Y. Wasserman, R. Schatzberger, and Y. Palti, “Disruption of cancer cell replication by alternating electric fields,” Cancer Res., vol. 64, no. 9, pp. 3288–3295, May 1, 2004.
- C. Wenger, R. Salvador, P. J. Basser, and P. C. Miranda, “The electric field distribution in the brain during TTFields therapy and its dependence on tissue dielectric properties and anatomy: A computational study,” Phys. Med. Biol., vol. 60, no. 18, pp. 7339–7357, Sept. 21, 2015.
- A. R. Korshoej, G. B. Saturnino, L. K. Rasmussen, G. von Oettingen, J. C. Hedemann Sørensen, and A. Thielscher. (2016, Oct. 3). Enhancing predicted efficacy of tumor treating fields therapy of glioblastoma using targeted surgical craniectomy: A computer modeling study. PLOS One. [Online].



