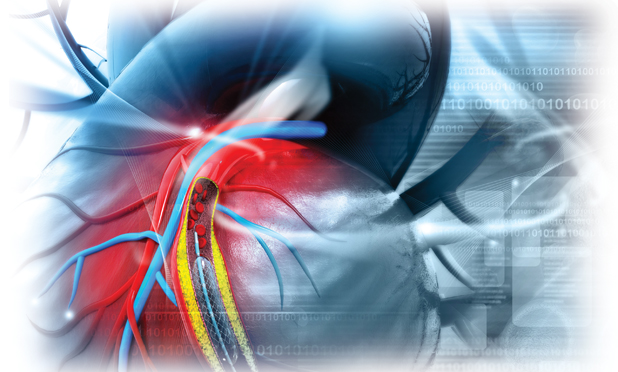
When an artery is blocked, stents are often the best way to open up the vessel. A mesh stent is tightly crimped over a tiny balloon and guided to the troubled spot; the balloon is inflated, expanding the stent, which forces the vessel open. Blood flow is restored.
These stents work well, but they aren’t perfect. For instance, a stent cannot always navigate the convoluted paths it must traverse to reach an occluded area, especially one that lies in a small branching vessel. In addition, a patient can sometimes develop a second narrowing of the artery—called restenosis—at the site of the implantation, and that can lead to a stroke or heart attack. To counter these limitations, researchers are developing new and better stent technologies that lower the risk of restenosis, allow stents to reach occlusions in tiny and remote vessels, and even detect restenosis much earlier.
Drug-Eluting Cardiac Stents

research and development fellow
at Boston Scientific, helped develop
Synergy, the company’s coronary-vessel
DES. She is working on the new Eluvia
drug-eluting vascular stent, which is
currently going through clinical trials.
(Photo courtesy of Boston Scientific.)
One of the ways clinicians reduce the risk of restenosis is through the administration of drugs that limit the formation of scar tissue or otherwise prevent the narrowing of vessels following stent implantation. With that in mind, researchers began developing drug-eluting stents (DESs), which carry medications and release the drugs exactly where they are needed. Next in line came projects to control the drug release so the patient received the medications in the proper amounts and for the correct time span.
“The research progress in DESs has been a natural evolution that combines many technological advancements to meet a number of unmet clinical needs,” says Yen-Lane Chen, a distinguished research and development fellow at Boston Scientific of St. Paul, Minnesota (Figure 1). She is also a key member of the multidisciplinary Boston Scientific team that developed a coronary-vessel DES, Synergy, which has been introduced in Europe and the United States over the past four years (Figure 2).
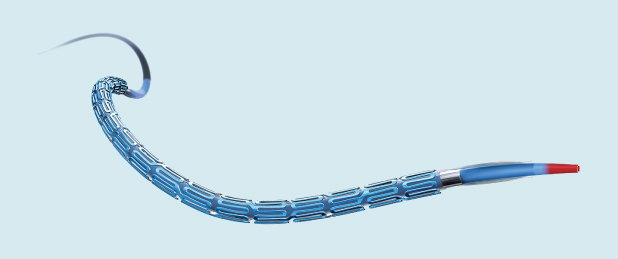
Synergy is notable in that its drug coating is only on the abluminal side of the stent, and its design facilitates the healing of the vessel after the implantation of the DES. The coating contains a poly(lactic-coglycolic acid), or PLGA, polymer that not only maintains the drug-coating’s integrity but also modulates its release out to approximately three months after stent implantation. At the same time, Chen explains, the polymer slowly degrades, eventually disappearing at the four-month mark [1]. The only thing remaining at the site after four months is the bare-metal stent.
An important requirement of all stents is that they be easy to maneuver through the twists and turns of vessels and across the blockage site. This maneuverability is especially important in treating complex lesions that are highly calcified or mostly occluded. To make their stents especially easy to guide through vessels, Boston Scientific scientists concentrated on the geometry of the stent’s scaffold, adjusting the connector angles and adding a laser-cut, stainless-steel “hypotube” to the stent-delivery system, Chen says. The result was an exceptionally flexible and thin stent, strong enough to be directed along the vessel path. She adds, “It was a combination of our new scaffold geometry design and system that gave us very good deliverability for Synergy.”
Although the stent is on the market, researchers continue looking for ways to improve it by making it even easier to implant, more economical, and, for some indications, stronger and thinner. Chen notes, “We are continuously improving the product.”
Thin Is In
Thin is indeed in, when it comes to DES design. “We are treating more and more complicated disease, such as multiple occluded, tortuous arteries and long blockages as well as blockages in smaller vessels, and one way to do that is to make the stent thinner so that it gets down the artery more easily and doesn’t take up too much space after it’s implanted,” says interventional cardiologist Matthew J. Price of Scripps Clinic in La Jolla, California (Figure 3). He led a large clinical trial of the exceptionally slim 2-mm Resolute Onyx, a cardiac stent developed by Medtronic of Dublin, Ireland. In early 2018, the U.S. Food and Drug Administration (FDA) approved the use of the stent, which delivers the immunosuppressant zotarolimus.

Numerous other thin stents are at various stages of development. Medinol of Tel Aviv, Israel, received FDA approval late last year for its slimprofile 2.5-mm EluNIR ridaforolimus-eluting stent [2], and other companies such as BioTronik of Berlin, Germany [3], and Biosensors International of Singapore [4] have their own superthin DES versions either on the market or waiting for approval to enter the market.
A thinner stent makes sense, but it does present some hurdles. One is visibility. Clinicians normally use X-ray imaging to track the stent as it is guided through blood vessels, but ultrathin stents can be so slim that they are invisible to X rays. “Ways to address that include changing the metal alloy in the stent scaffold, such as impregnating the stents with platinum, chromium, or other alloys, to make them brighter under X rays,” Price says. The Resolute Onyx, for instance, was designed with a platinum iridium core and a cobalt alloy shell, which enhance its radiopacity while also maintaining the stent’s slim, strong, and flexible characteristics, he explains.
Thin stents do seem to work, Price notes. In the clinical study of the Resolute Onyx, specifically the 2.25- to 4-mm size, researchers collected periodic angiograms of patients following placement of the stents: one at the time of stent implantation, another at the nine-month mark, and one more at 21 months to identify any narrowing. “We demonstrated that the stent worked just as well as, if not better than, the predicate version of the stents, and these data were used to help obtain its FDA approval,” he says.
A separate Medtronic-funded trial tested the 2-mm Resolute Onyx in patients whose occlusions required ultrathin stents, and it showed that a very low number of stent-related complications at one year: target lesion revascularization was 2%, and target vessel myocardial infarction was 3%. No episodes of stent thrombosis occurred [5].
“We’re getting closer and closer to the point that we can treat almost any blockage with incredibly good results and long-term safety,” Price notes. “It’s going to become increasingly challenging to show that these technologies are better than what we currently have because we’ll need larger and larger trials, but every couple of years, we take one step closer.”
Beyond Cardiac Stents
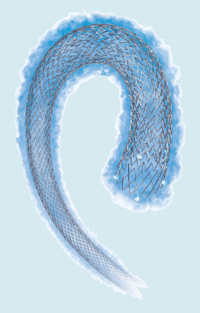
drug-eluting vascular stent is designed
for peripheral vessels, such as those
in the legs. Stents placed in a leg must
handle heightened levels of mechanical
stress and be able to deliver drugs over
a 12-month period. Eluvia is currently
undergoing clinical trials. (Illustration
courtesy of Boston Scientific.)
The peripheral vessels, especially those of the legs, would benefit from effective stents too. These could help the huge over- 50 population who have peripheral arterial disease (PAD). According to the U.S. National Heart, Lung, and Blood Institute, one in every five Americans has PAD [6], which not only increases the risk of heart attack and stroke but can also come with occlusions that are painful or limit movement.
One stent being developed to tackle this problem is Boston Scientific’s Eluvia drug-eluting vascular stent, currently undergoing clinical trials (Figure 4). “The coronary vessels are different from the peripheral vessels, so we had to start from the very beginning and understand the disease etiology and the vessel properties, as well as the challenges of stent material,” Chen explains. “For one thing, we are constantly moving our legs, so we had to come up with the right stent material and the right design to be able to sustain that level of mechanical stress and not fracture. And we also had to find a way to deliver the longer duration of drug-elution time that is needed for this application.”
Their answer was a self-expanding nitinol stent platform composed of a polymer, made of poly(vinylidene fluoride) and hexafluoropropylene, as well as the antiproliferative drug paclitaxel [7]. The drug–polymer pairing in the Eluvia stent provides controlled and sustained release of the medication for 12 months to match the needs of peripheral vascular interventions, according to Chen.
Studies have shown that the Eluvia stent has a primary patency rate of 96.4% in the first year, so the vast majority of patients needed no additional intervention for the occlusion during the 12 months following the stent placement. In two years, the primary patency rate was 83.5% [8]. This compares to some competing products that had 20% primary patency rates in the first year, she says.
Eluvia is already available in Europe and is in the midst of a clinical trial in the United States. Chen adds, “Our hope is that we will receive FDA approval by next year.”
Spotting Restenosis Early
Another way to make stents more effective is to use them as monitoring tools, says microsystems researcher Kenichi Takahata, Ph.D., P.Eng., associate professor in the University of British Columbia’s Department of Electrical and Computer Engineering (Figure 5). “Although stents are used in millions of patients every year, there is currently no diagnostic procedure or technology to pick up the issue of restenosis at an early stage,” he contends. The first sign of a coronary restenosis, for instance, is typically the patient’s complaint of discomfort in the chest, “but by that point, the narrowing has progressed quite a bit already,” he asserts.

In an effort to address this problem, Takahata and his laboratory group are turning to microelectromechanical systems and wireless technologies to make a smart stent [9]. Specifically, the group developed an implantable micropressure sensor (Figure 6) that monitors even slight changes in blood-flow pressures, thereby detecting the beginning signs of restenosis. “Next, we needed to have an interface for the device so we could send any warning about flow changes to the patient and/or the clinician,” he says. “So we designed our stent to work as a radio-frequency antenna. This ‘stentenna’ could send the flow information in a wireless manner to a reader system.”
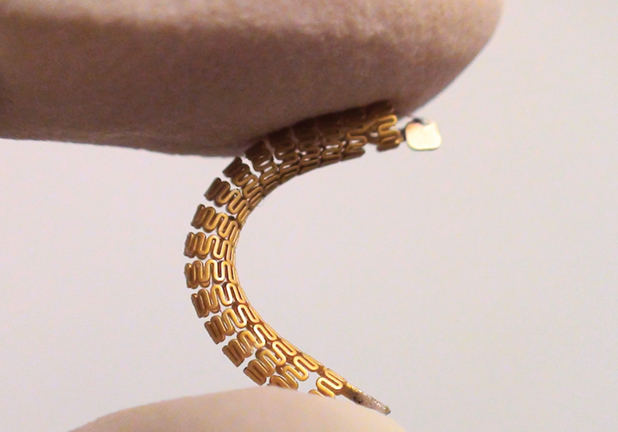
A major consideration for the project was making the stent compatible with standard clinical procedures for implantation so doctors could switch to the new smart stent without having to learn any new procedures. “To be compatible, we had to design the device to be very robust, because implantation is a mechanically demanding process,” explains Takahata. To do that, the researchers constructed both the stent and the sensor of medical-grade stainless steel and employed some fancy microfabrication, including specially chosen techniques to ensure both could survive the normal crimping and expansion stents undergo. To fabricate the tiny pressure sensor out of the steel, researchers used a technique Takahata calls microelectrodischarge machining. To bond the sensor to the stentenna, researchers employed laser microwelding.
The group has completed bench and animal testing to verify that the smart stent can survive the implantation process. The group has also demonstrated its wireless-communication capability. The next steps are additional animal testing of the prototype, to be followed by a human clinical trial. Takahata holds the patents on the smart stent and is now searching for an industry partner to pursue its commercialization.
While that proceeds, he and his group are working on further miniaturizing the sensor and designing a practical manufacturing process. “In the future, we also hope to develop the reader system into a smaller device, so that the monitoring can be done through a handheld unit; for example, in a form of cell phones possibly,” he adds, noting that they are extending their smart-stent technology to other medical areas where stents play a critical role but suffer from similar clogging issues. He remarks, “The application range could be pretty broad.”
On the Horizon
While work proceeds on these stents, another segment of the field—fully bioabsorbable stent scaffolds—is dealing with the effects of a setback in 2017, when Abbott Vascular removed its version from the European and U.S. markets amid low commercial sales and concerns about thrombosis rates [9, 10]. That put a damper on all work in this research area, Chen remarks, and Boston Scientific put its own project on hold too. Once the negative publicity dies down a bit, Chen thinks the field will likely move toward fully absorbable stent scaffolds once again, although she can’t predict how soon that may be.
“The concept of fully bioabsorbable scaffolds generated a lot of early enthusiasm because there is no need for a scaffold after a certain period of time,” she says. “It’s very reasonable—very intuitively obvious—that we would want the scaffold to disappear after its usefulness is over, so there are still some true believers about the fully absorbable scaffold. We just have to wait and see how the market dynamic evolves.”
In the meantime, researchers can find other options to improve stent technology, Chen contends. “There’s still plenty of room for multidisciplinary innovation. We need people with all skills—mechanical engineering, chemical engineering, material sciences, pharmaceutics, chemistry, and computer modeling. It truly takes a village of people from various disciplines to work together to advance medical-device technology and better treatment for the patients.”
References
- G. J. Wilson, A. Marks, K. J. Berg, M. Eppihimer, N. Sushkova, S. P. Hawley, K. A. Robertson, D. Knapp, D. E. Pennington, Y.-L. Chen, A. Foss, B. Huibregtse, and K. D. Dawkins, “The SYNERGY biodegradable polymer everolimus eluting coronary stent: Porcine vascular compatibility and polymer safety study,” Catheterization Cardiovascular Interventions, vol. 86, no. 6, pp. E247–E257, Nov. 15, 2015.
- U.S. Food and Drug Administration. (2018). EluNIR® ridaforolimus eluting coronary stent system. [Online].
- BioTronik. (2018). [Online].
- Biosensors International. (2018). [Online].
- U.S. National Heart, Lung, and Blood Institute. (2018). Facts about peripheral arterial disease (P.A.D.). [Online].
- M. J. Price, S. Saito, R. A. Shlofmitz, D. J. Spriggs, M. Attubato, B. McLaurin, A. Popma Almonacid, S. Brar, M. Liu, E. Moe, and R. Mehran, “First report of the Resolute Onyx 2.0 mm zotarolimus-eluting stent for the treatment of coronary lesions with very small reference vessel diameter,” J. Amer. College Cardiology Cardiovascular Interventions, vol. 10, no. 14, pp. 1381–1388, July 24, 2017.
- S. Müller-Hülsbeck, “Eluvia™ peripheral stent system for the treatment of peripheral lesions above the knee,” Expert Opinion Drug Delivery, vol. 13, no. 11, pp. 1–6, Aug. 2016.
- S. Müller-Hülsbeck, K. Keirse, T. Zeller, H. Schroë, and J. Diaz-Cartelle, “Long-term results from the MAJESTIC trial of the Eluvia Paclitaxel-Eluting Stent for femoropopliteal treatment: 3-year follow-up,” CardioVascular Interventional Radiology, vol. 40, no. 12, pp. 1832–1838, Dec. 2017.
- X. Chen, B. Assadsangabi, Y. Hsiang, and K. Takahata, “Medical implants: Enabling angioplasty-ready ‘smart’ stents to detect in-stent restenosis and occlusion,” Advanced Sci., vol. 5, no. 5, May 2018.
- M. J. Lipinski, R. O. Escarcega, N. C. Baker, H. A. Benn, M. A. Gaglia, Jr., R. Torguson, and R. Waksman, “Scaffold thrombosis after percutaneous coronary intervention with ABSORB bioresorbable vascular scaffold: A systematic review and meta-analysis,” J. Amer. College Cardiology Cardiovascular Interventions, vol. 9, no. 1, pp. 12–24, Jan. 2016.
- Abbott. (2018). ABSORB GT1 bioresorbable vascular scaffold. [Online].