By changing the electrical signaling of neurons in the brain using light, optogenetics is revealing novel treatments for hearing loss and other neurological disorders
In 2005, Edward Boyden of Stanford University and Karl Deisseroth of MIT opened up a new field of research—optogenetics—that offered the potential to revolutionize the treatment of neurological conditions. Their quest to genetically encode brain cells to be photosensitive has unlocked a toolkit that researchers are adding to consistently, and the implications for neuroscience and neuroengineering are vast.
Their work primarily centered on the importance of opsins—the universal photoreceptor molecules of all visual systems in the animal kingdom. Opsins are light-sensitive proteins that change their structure from a resting state to a signaling state when they absorb light. Boyden and Deisseroth found that channelrhodopsins, which allow the fast depolarization of neurons when exposed to light, can be transduced into neurons to enable the regulation of electrical signaling. In essence, the use of different opsins can be used to control the excitation or inhibition of neurons and, therefore, affect brain function. Since that potential was first identified, research in the field has blossomed, leading to the identification of many new opsins and the investigation of myriad applications.
“Optogenetics has continued to thrive and the major drivers include the discovery and engineering of new opsins—the engineering of appropriate vectors, promoters, and other genetic tools for efficient and specific opsin expression,” says Tobias Moser, professor of auditory neuroscience at Göttingen University (Figure 1). “The engineering of optical stimulators, a vivid and broad range of applications in life sciences, and a focused and serious effort toward clinical translation of some applications has emerged,” he adds. “Optogenetics has the potential to replace clinical applications of electrical stimulation where selectivity and specificity are required, and to generate new treatment options that cannot be implemented by current medical devices or drugs.”

Figure 1. Tobias Moser, professor of auditory neuroscience at Göttingen University, studies the use of optogenetics in hearing restoration. (Photo courtesy of Tobias Moser, Göttingen University.)
Hearing with light
One advantage of optogenetics is the specificity and precision with which the timing, location, and intensity of signals in the brain can be controlled using light. Furthermore, it is a relatively inexpensive method of brain stimulation, as it uses light-emitting diodes (LEDs) or lasers as the light source. While it is still in the early stage of development for the treatment of human diseases, researchers are exploring many diverse applications and the results from testing in rodents are, in many cases, promising.
Moser’s work on treating deafness is a prime example. In recent years, deafness is increasingly treated using cochlear implants (CIs)—small but highly sophisticated electronic devices consisting of an external microphone and speech processor fitted behind the ear and a surgically implanted receiver beneath the skin. The first CI was approved by the U.S. Food and Drug Administration in the 1980s, and The National Institute on Deafness and Other Communication Disorders reports that nearly 737,000 devices had been implanted globally by 2019.
“Today’s cochlear implants are the most successful neuroprosthetic to date,” Moser explains. “Yet they make limited use of the neurons available for sound encoding in the cochlea. Because the frequency selectivity of electrical stimulation is limited—typically less than ten independent channels are available no matter how many electrodes are provided—the quality of artificial hearing is limited, too.”
“The natural process of hearing, in which hair cells trigger precise points on the cochlear nerve, can be thought of as playing the piano with your fingers,” he adds. “Cochlear implants are more equivalent to playing with your fists.” The result is that, even with a year of training, most CI users can understand speech in quiet environments, but often fail to understand in a noisy environment. Furthermore, it is difficult to track melodies be it in speech or in music.
Optogenetic hearing restoration combines gene therapy and an optical CI. Moser saw optogenetics as the best route to improve outcomes because optical stimulation can overcome the bottleneck of electrical stimulation (Figure 2). “As light can be better confined in space, optogenetic CIs promise to improve the spectral selectivity,” Moser explains. “This means that the number of independent channels can be increased. Claus-Peter Richter of Northwestern University proposed the direct stimulation of cochlear neurons with high-energy infrared light pulses. Optogenetics helps to bring the energy budget of optical stimulation closer to that of electrical stimulation.”
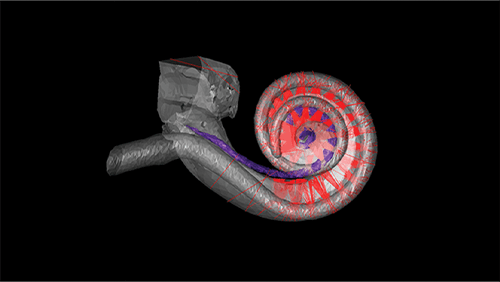
Figure 2. Human hearing depends on a snail-shaped structure called the cochlea in the inner ear. A new kind of CI for people with disabling hearing loss would use beams of light to stimulate the cochlear nerve. Image shows an optical ray-tracing simulation of 3-D model of a human cochlea with 32-emitter optical CI. (Photo courtesy of Lakshay Khurana, Institute for Auditory Neuroscience, University Medical Center Göttingen and Max Planck Institute for Multidisciplinary Sciences, Göttingen.)
While there are many stages to go before Moser can test his concept in humans (Figure 3), he hopes to see evidence of efficacy and safety in nonhuman primates, followed by a first-in-human (FIH) trial by 2026. Nevertheless, his work has already opened new doors in this field of research. “This application of optogenetics to the auditory system has already contributed substantially to advancing optogenetics in general, be it by the development of ultrafast channelrhodopsins, the optimization of viral gene transfer, or the development of multichannel optical stimulators,” he remarks.

Figure 3. Optogenetic hearing restoration builds upon a combination of a gene therapy and the optical CI as a medical device. (Photo courtesy of Dr. Daniel Keppeler, Institute for Auditory Neuroscience, University Medical Center Göttingen and Max Planck Institute for Multidisciplinary Sciences, Göttingen.) Reprinted from doi: 10.15252/emmm.202215798.
Closed-loop control
In the U.K., another project is rapidly adding to the optogenetics toolkit. In Controlling Abnormal Network Dynamics using Optogenetics (CANDO), a team of more than 30 neuroscientists, engineers, and clinicians based at Newcastle University, Imperial College London, University College London, and The Newcastle Hospitals NHS Foundation Trust aims to develop a cortical implant for optogenetic neural control that will be used for an FIH trial in patients with focal epilepsy.
As with many neurological diseases, focal epilepsy is caused by the disruption of rhythmic activity, i.e., brain waves, and the abnormal activity can often be localized to a small ‘focus,’ which then spreads and causes seizures. Epilepsy affects 600,000 people in the U.K. and almost one-third of cases fail to respond to conventional drug treatments. In such cases, surgical removal of the focus may be required. “There have been many advances in brain interface technology recording signals from the brain and in neurostimulation delivering signals into the brain,” remarks Prof. Andrew Jackson, Newcastle University, one of the founders of the CANDO project. “For years I have looked at linking those together to control stimulation of the brain through a closed-loop feedback mechanism.”
“We chose to focus on focal epilepsy because it is a good example of a neural network out of control,” he adds. “The build-up of oscillatory patterns of activity can lead to a seizure with deleterious effects on the functioning of the brain, so a closed-loop feedback approach could stabilize the seizure oscillation. We had a mechanistic idea of how optogenetics could control this, so a place where oscillatory dynamics have gone wrong is a good place to start.”
The possibility of neurosurgical resection for people with drug-resistant epilepsy makes the use of a novel gene therapy viable despite the risks of the accompanying implant. The worst outcome is resection—the surgical pathway that might otherwise have been considered in the first place.
“The problem part of the brain can be well localized in focal epilepsy,” Jackson explains. “The strategy could be to take a case where part of the brain could be resected without complications. An ethical development pathway emerges in a part of the brain that would otherwise be cut out, though we are still a long way from proposing a trial like that in humans.” “The problem is that electrical stimulation is indiscriminate,” he adds. “It stimulates all neurons in a given region and it produces many electrical artifacts. Optogenetics using cell-type specific promoters can get opsins into specific cell types, so recording, which is done electrically, and stimulation, which is done optically, have different modalities and there is no interference.”
Undoubtedly, there are many risks and many challenges that arise with optogenetics. For Jackson’s proposed method of closed-loop control—where feedback on the brain’s activity would automatically trigger the appropriate signals to control brain function—there is a neurosurgical element, but gene therapies also carry risk. “The biggest unknowns are on the gene therapy side but there are big engineering challenges, too, such as how you get light into the tissue,” Jackson observes. “We chose to use LEDs and there are no other therapies where LEDs are long-term implanted in the brain, so we need to look at safety processes and the effects of light in the brain.”
Furthermore, existing deep brain stimulators are similar in design to pacemakers, with electronic components housed in an airtight titanium container and a simple lead taking current to the brain. The CANDO implant would contain control circuitry and the LEDs in the brain implant itself, so the encapsulation process must be proven.
“The bigger challenge is that, building on the basis of a lot of successful experiments in rodents, we now have to scale that up to a larger brain,” Jackson adds. “The amount of light that is needed to control a proportion of the rodent brain—a single LED or a small light fiber—is different to the amount needed for a larger brain, so we need to ensure we are expressing enough to stimulate a large enough volume of neurons.”
An idea with a bright future
Both CANDO and Moser’s project are pushing back the boundaries of optogenetics, in which the development of new opsins is rapidly accelerating. Recent work on kalium channelrhodopsins as natural light-gated potassium channels that mediate optogenetic inhibition, for example, is approaching what Jackson calls “the Holy Grail of neuron inhibition.”
The treatment of neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease is an obvious target for optogenetics, but the concept is being applied to other excitable tissues in the human body, which could lead to treatments for cardiovascular disease and other conditions.
Whether individual projects such as CANDO lead to successful outcomes or not, they will greatly advance the understanding of how optogenetics can be applied and what challenges it presents. Jackson is hopeful, for example, that development of closed-loop control mechanisms using real-time feedback in the human brain will be accelerated by his work. Optogenetics is clearly a field of research with a bright future, and perhaps its only limit is what the brain can imagine.