Every year, hundreds of thousands of children worldwide are born with sickle cell disease, a genetic disorder that impacts the hemoglobin molecules in blood. If left undiagnosed and untreated, most affected children will die before reaching the age of five. However, highly accurate diagnostic methods and effective treatment regimens for sickle cell disease have been known for many years, and children who receive early diagnosis and subsequent comprehensive care survive well into adulthood—as evidenced by the tremendous success of universal newborn screening programs in North America and Europe.
Why, then, does sickle cell disease continue to constitute a significant portion of the mortality rate of children under five in many countries? The primary problem is that laboratory methods for diagnosing sickle cell disease, while highly sensitive and accurate, are too complicated, costly, slow, laborious, and equipment- and electricity-dependent for widespread use in resource-limited settings. This lack of access to diagnostic methods has created a huge disparity in expected outcomes between children with sickle cell disease born in resource-rich countries and those born in resource-limited settings, such as sub-Saharan Africa, where the incidence of sickle cell disease is actually highest.
Importantly, the treatments necessary to reduce early childhood deaths due to sickle cell disease are inexpensive, practical, and already available in most resource-limited settings. Prophylactic oral penicillin, immunization against pneumonia, mosquito bed nets, and parental education about symptom management have all been shown to significantly improve outcomes for children with sickle cell disease. It is not, therefore, impracticality or lack of access to treatment but rather the inability to accurately identify affected children in need of early care that currently prevents effective sickle cell disease management in resource-limited settings.
There is, consequently, an urgent need for a diagnostic test that is sufficiently inexpensive, portable, simple, and rapid to enable screening for sickle cell disease in resource-limited settings where established infrastructure, specialized equipment, and trained personnel are often lacking. Previous attempts at creating point-of-care tests for sickle cell disease have taken advantage of the fact that sickle hemoglobin, the abnormal form of hemoglobin found in individuals with sickle cell disease, polymerizes and becomes insoluble in low-oxygen conditions. These tests are intended to enable health workers determine whether sickle hemoglobin is present in a blood sample by visually assessing the “cloudiness” (i.e., the amount of insoluble sickle hemoglobin) of a mixture of blood and hemoglobin solubility buffer. However, these solubilitybased tests are not quantitative, cannot be used for newborns or young children, are notoriously difficult to interpret, are subject to interference from numerous common conditions, and have diagnostic accuracies that are little better than that of a coin toss. Therefore, conventional approaches do not adequately address the urgent need for high-quality point-of-care sickle cell testing.
How Can a Piece of Paper Help?
As with most research projects, it all started with a few too many cups of coffee. Inspiration struck Sergey Shevkoplyas (an author of this article), as he looked at the paper napkin he used as an improvised coaster for his coffee. The napkin was covered in overlapping coffee stains with light centers and dark edges. The intricate pattern brought to mind several scientific papers he had read describing the physics behind why the tiny brown particles that give coffee its color move outward, toward the edge of the stain, as spilled coffee dries. He then thought back to the existing solubility-based point-of-care tests for sickle cell disease and wondered if paper and the coffee stain effect could be utilized to separate clumps of polymerized sickle hemoglobin from the rest of the mixture.
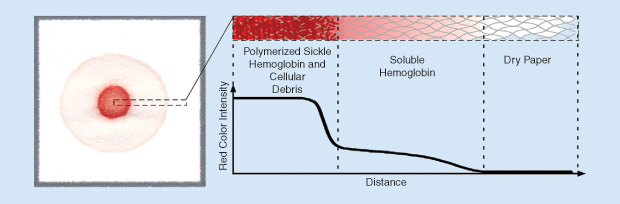
After testing out this idea in the laboratory, we soon had a working prototype. The biochemistry of this new test was similar to that of existing point-of-care tests (i.e., mixing blood with hemoglobin solubility buffer causes sickle hemoglobin to polymerize and fall out of solution). But, by depositing a drop of this in overlapping coffee stains with light centers and dark edges. The intricate pattern brought to mind several scientific papers he had read describing the physics behind why the tiny brown particles that give coffee its color move outward, toward the edge of the stain, as spilled coffee dries. He then thought back to the existing solubility-based point-of-care tests for sickle cell mixture on chromatography paper (a porous cellulose material similar to a paper napkin), we found that insoluble clumps of polymerized sickle hemoglobin became entangled in the paper fibers where the drop was deposited, while other soluble forms of hemoglobin wicked laterally outward through the paper pores. The natural red color of hemoglobin resulted in the formation of blood stains having an easily recognizable pattern related to the amount of sickle hemoglobin in the blood sample without the need for additional signal amplification steps (Figure 1).
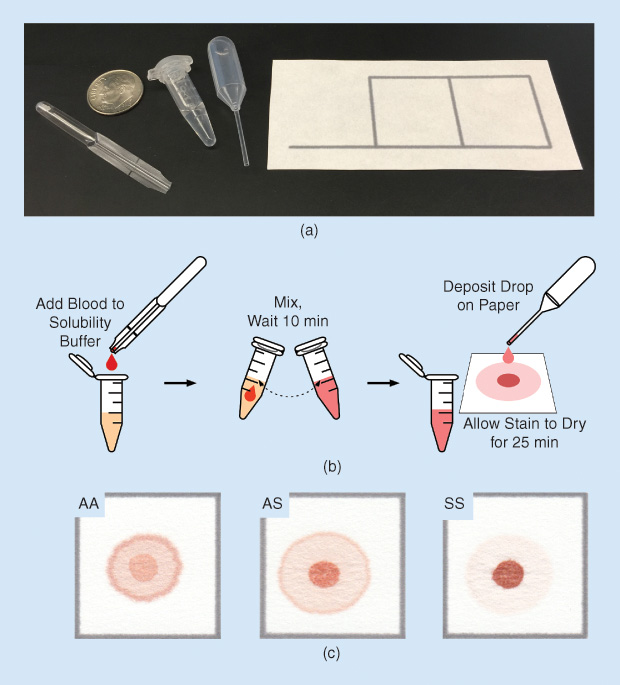
To validate this new diagnostic method, we tested blood samples from individuals with sickle cell disease, sickle cell trait (carriers of a single copy of the gene for sickle hemoglobin), and normal hemoglobin. We found that the color intensities of the center spot and peripheral ring of the blood stain were proportional to the concentrations of sickle and normal hemoglobin, respectively. Stains for normal individuals were nearly uniformly pink throughout, patterns for individuals with sickle cell disease had dark red center spots and very faint peripheral rings, and patterns for individuals with sickle cell trait were intermediate, with moderately red center spots and moderately pink peripheral rings [Figure 2(c)]. Whether evaluated visually by eye or via automated image analysis, the test was found to be capable of highly sensitive and specific differentiation among normal, sickle trait, and sickle cell disease [1], [2].
The Power of Paper
The paper-based sickle cell disease test combines the simplicity and low cost of point-of-care tests with the precision and accuracy of laboratory-based methods. The test uses only disposable plastic droppers and tubes, it does not require any specialized equipment or access to electricity, it can be performed and interpreted in approximately half an hour, and it costs less than US$0.10 in materials per test. Also, the reagents used in the hemoglobin solubility buffer are food grade and stable at room temperature, meaning that the test does not require refrigeration during shipping or storage (which is a prerequisite for tests intended to be used in resource-limited settings, where maintaining a temperature-controlled supply chain may be next to impossible).
The test was also designed to be exceedingly simple: its performance requires only basic skills, similar to those needed for cooking (e.g., mix, wait, drop, look). A user with no prior experience can be trained to perform and interpret the test with expert-level proficiency in under one hour, and test results remain stable for days to weeks after the test is completed. Test performance has also been shown to remain consistent between tests given by skilled users in laboratory settings and health workers in resource-limited clinical settings (Figure 3).
A simple, low-cost, and inherently portable test for sickle cell disease has exciting implications for screening programs in resource-limited settings. The lower cost per test means that existing screening programs can increase the number of individuals tested or choose to focus limited resources on improving care for identified individuals with sickle cell disease. Additionally, by removing the need for equipment, electricity, and specially trained personnel, the paper-based test enables screening efforts to move outside of centralized hospitals and clinics and capture the large number of out-ofhospital births that occur at home or in remote health centers (see “Traveling to Angola to Validate a Paper-Based Sickle Cell Disease Test”).
[accordion title=”Traveling to Angola to Validate a Paper-Based Sickle Cell Disease Test”]
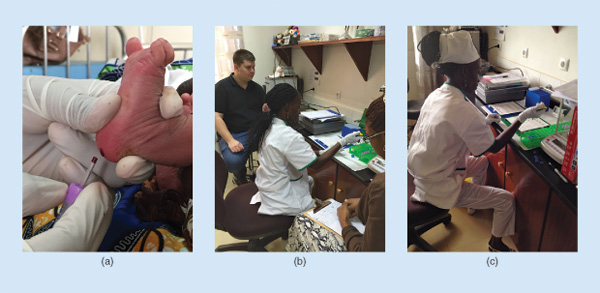
When I started graduate school, I had no idea that it would take me halfway around the world to a small clinic in Cabinda, Angola, but I’m glad that it did. Since 2011, I have been a part of the team of engineers and clinicians, led by Dr. Sergey Shevkoplyas, working to develop a simple, low-cost, paper-based test for sickle cell disease— a common inherited blood disorder that is most prevalent in sub- Saharan Africa (Figure S1).
In 2014, our clinical collaborator Dr. Alex George of Texas Children’s Hospital and I traveled to the Angola Sickle Cell Initiative Clínica das Células Falciformes (Sickle Cell Clinic) and the Primero de Maio Obstetric Hospital in Cabinda, Angola, to determine whether the paper-based test we had developed was actually useful in a real-world, resource-limited clinical setting.
The sickle cell clinic is a part of the Angola Sickle Cell Initiative, a joint program among Texas Children’s Hospital, Baylor College of Medicine, the Angolan Ministry of Health, and Chevron. Currently, local health workers use isoelectric focusing, a conventional laboratory-based method of diagnosing hemoglobin disorders, to screen newborns for sickle cell disease. This approach to testing has enabled them to identify thousands of affected newborns but is not without limitations.
A limiting factor that became apparent during the visit was electricity. Blackouts are unfortunately common in Cabinda— during our two-week visit, the power was off more often than it was on—and, when the power is off, isoelectric focusing simply can’t work. Worse yet, if the electricity cuts out in the middle of the process, the gel has to be discarded and the process repeated, which means the loss of costly materials, hours of work, and, most importantly, patient samples.
This is where the paper-based test really began to shine. When the power went out and the health workers realized that they could keep using the paper-based test regardless, they exclaimed that they loved the new test and that it was much better than their current machines. It was amazing to see how much better a simple test that had been engineered with limited resources in mind worked in this setting, as compared with more complex technologies designed for use in a well-equipped hospital laboratory.
There is only so much you can plan for beforehand in the laboratory; you never really know what will work and what won’t until you’re out in the field. We expected that things like high ambient temperature and humidity would affect blood stain drying time. But we couldn’t have anticipated that the biggest problems would be caused by commercially available blood collection tubes, which proved difficult for the health workers (who were used to collecting dried blood spots on paper) to label and use. The feedback from the Angolan health workers we taught to use the test proved to be invaluable in making the test robust and user friendly.
The time spent in Angola guided our approach to optimizing and commercializing the paper-based test and will continue to shape my design thinking for years to come. I hope to continue to use what I learned in Angola to translate healthcare products from the bench to the bedside and to increase access to laboratoryquality health care in resource-limited settings.
The unofficial motto of the Shevkoplyas laboratory is that we should always make what we do matter. In the months following our trip to Angola, the health workers there have screened more than 700 mothers and their newborns for sickle cell disease using the paper-based test, and to date the paper-based test has correctly identified every single newborn with sickle cell disease. I’d say that definitely matters.
—Nathaniel Z. Piety
[/accordion]
Beyond Diagnosing Sickle Cell Disease
The “lab-on-a-sheet” approach used to create the paper-based sickle cell disease test provides a robust platform for the development of ultra-low-cost diagnostic and quantitative tests. Chromatography paper provides a means for lateral wicking of liquids through the thickness of the paper as well as a mechanism for size- and shape-dependent separation of soluble and insoluble components within the liquid, all without requiring external equipment, electricity, or running water.
In addition to diagnosing sickle cell disease, we have expanded the paper-based lab-on-a-sheet platform to enable quantification of sickle hemoglobin percentage [3], measurement of hemoglobin concentration, separation of plasma from whole blood, and detection of fetal levels of sickle hemoglobin in newborns. Nathaniel Piety, a Ph.D. student in Shevkoplyas’ laboratory (also an author of this article), has recently been awarded an American Heart Association predoctoral fellowship to develop a quantitative sickle hemoglobin test capable of enabling near- real-time measurement of sickle hemoglobin during blood transfusion therapy.
Toward Universal Screening
Shevkoplyas has cofounded a company, Halcyon Biomedical Incorporated, that aims to commercialize the paper-based test as well as other technologies developed in his laboratory. In 2014, the National Heart, Lung, and Blood Institute of the National Institutes of Health awarded Halcyon Biomedical Incorporated a two-year, US$450,000 Phase I Small Business Innovation Research grant to continue commercialization of the paper-based sickle cell disease test; in September 2016, another US$1 million in Phase II funding was granted to complete clinical validation of the paper-based test at several sites throughout sub-Saharan Africa.
Acknowledgments
We would like to acknowledge support from the Baylor College of Medicine Center for Global Initiatives; the Angola Sickle Cell Initiative; the National Heart, Lung, and Blood Institute of the National Institutes of Health (R01HL117329, R43HL123809); and Halcyon Biomedical Incorporated.
References
- X. Yang, J. Kanter, N. Z. Piety, M. S. Benton, S. M. Vignes, and S. S. Shevkoplyas, “A simple, rapid, low-cost diagnostic test for sickle cell disease,” Lab Chip, vol. 13, no. 8, pp. 1464–1467, 2013.
- N. Z. Piety, X. Yang, J. Kanter, S. M. Vignes, A. George, and S. S. Shevkoplyas, “Validation of a low-cost paper-based screening test for sickle cell anemia,” PLoS ONE, vol. 11, no. 1, p. e0144901, 2016.
- N. Z. Piety, X. Yang, D. Lezzar, A. George, and S. S. Shevkoplyas, “A rapid paper-based test for quantifying sickle hemoglobin in blood samples from patients with sickle cell disease,” Amer. J. Hematol., vol. 90, no. 6, pp. 478–482, 2015.



