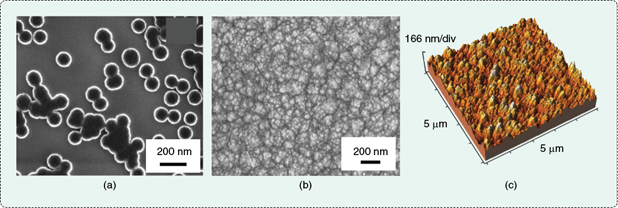
Transforming diamond sizes from the micron regime into a few billionths of a meter probably doesn’t increase their value in jewelry, but it certainly adds an extraordinary value in numerous physical science, engineering, and medical applications. Nanodiamonds are generally defined as synthetic diamond materials that have crystalline or feature sizes (e.g., grain or particle sizes) between 1 and 100 nm (10–9–10–7 m) and include two forms: particulate (zero-dimensional) and thin film (two-dimensional). Figure 1 shows particulate nanodiamond (PND) and nanodiamond thin films under a scanning electron microscope (SEM) and atomic force microscope (AFM). Because the nanodiamond thin film is actually composed of nanosized columnar crystals or grains, the material is usually called nanocrystalline diamond (NCD) or ultra-NCD (UNCD, grains sizes <10 nm).
Historically, the utilization of crystalline diamond in science and engineering has constantly encountered cost problems. But owing to the fast development of chemical vapor deposition (CVD) and denotation techniques, the manufacture of nanodiamonds from inexpensive sources is already achievable in a cost-effective manner. For example, the fabrication of a 200-nm-thick NCD thin film costs about US$1/cm2 (equipment cost not included) in a CVD research laboratory while industrial production would further lower this cost. This cost reduction has led to numerous studies and diverse applications of nanodiamonds at an increasing rate in the past few years.
Nanodiamonds possess superior mechanical and tribological properties comparable to bulk single-crystalline diamond (e.g., natural diamond), such as extremely high strength and hardness, superior wear resistance, and high inertness. More importantly, because of nano or quantum effects, nanodiamonds exhibit several new properties, which do not exist in single crystalline or micron crystalline diamonds. These properties include tunable conductivity (note that the conventional diamond is an insulator), field electron emission properties, conformal coating feasibility, and greater biocompatibility. The combination of these extraordinary nanodiamond properties opens new avenues for detecting, imaging, and controlling as well as manipulating biomolecules, cells, and microbes, leading to a broad range of potential new applications in biology, medicine, and health care engineering.
In this article, research advances and the progression of nanodiamonds in biomedical applications are reviewed with a particular interest given to their role in tissue regeneration/repair, drug delivery, and biosensing. Two types of nanodiamonds, PND and NCD thin films, are discussed here.
Nanodiamonds for Bone Regeneration
Orthopedics is one of the primary fields where nanodiamonds have been extensively considered, and both PNDs and NCDs have demonstrated a large potential for tissue regeneration or repair. Some studies even suggest that nanodiamonds may be more biocompatible than other carbon nanostructures such as carbon blacks, fullerenes, and carbon nanotubes [1]. Because of their structural nature, PNDs and NCDs have been studied and developed for different applications of bone regeneration. PNDs are frequently combined with other materials such as polymers to produce nanocomposite bone scaffolds, while NCD films are usually used as implant coating materials to promote both mechanical (mainly antiwear) and osseointegrative properties. These two directions are summarized below.
![FIGURE 2 Examples of nanodiamonds used for bone tissue engineering: (a) the SEM image of a porous octadecylamine-functionalized PND/PLLA nanocomposite, (b) the clear fluorescence of the nanocomposite scaffold seen in the background of osteoblasts after three days of culture (modified from [2] with permission) and (c) enhanced osteoblast proliferation on –NH2 modified NCD films (with higher surface energy) compared to (d) osteoblast proliferation on unmodified NCD films (with lower surface energy).](https://www.embs.org/wp-content/uploads/2014/03/31498.png)
PNDs have been added to biocompatible polymers to increase material hardness and Young’s (elastic) modulus as well as to provide additional functionalities such as imaging, sensing, and tissue growth abilities. Multifunctional bone scaffold materials have been developed using biodegradable polymers such as poly (l-lactic acid) (PLLA) and surface-modified PNDs, as an example shown in Figure 2(a) [2]. The addition of small amount of PNDs results in great enhancements in the Young’s modulus, hardness, strain at failure, and fracture energy of the scaffold materials compared to polymers alone. These enhancements render the mechanical properties of the scaffolds close to that of human cortical bone. Moreover, the surface-modified PND can also provide bright fluorescence for medical imaging [Figure 2(b)] and more options for drug loading and delivery. In vitro cell tests have also shown that PND/polymer scaffold supports osteoblast (bone-forming cell) growth and differentiation, and enhances biomineralization and formation of bonelike apatite on the scaffold in simulated body fluid (SBF). These results indicate that PND/polymer nanocomposite scaffolds are potentially useful for a wide range of orthopedic regenerative engineering applications.
The application of PND as a bone implant coating, however, has not been extensively studied to date. There have only been a few recent studies showing that detonation synthesized PND can stimulate the precipitation of calcium phosphate or hydroxyapatite (HA) on titanium (Ti)–copper (Cu) alloys or stainless steel when immersed in a mixture of SBF and PND suspensions [3]. This demonstrates an osteoconductive property of PND and its possible application in fabricating calcium phosphate or HA coatings on metallic implants.
In contrast, because of its feasibility and ease for deposition onto various substrate materials (e.g., Ti and its alloys, stainless steel, silicon, and ceramics) with suitable adhesion strength, NCD films have been mainly studied as implant coatings to provide wear- and corrosion-resistance as well as tissue regenerative functions. In the past few years, osteoconductive and osteogenic properties of NCD films have also been evaluated and investigated, mostly by in vitro studies. For instance, osteoblast adhesion and proliferation assays on NCD (or UNCD) in comparison to platinum, silicon, or borosilicate glass have revealed that NCD has superior cytocompatibility properties, such as increased cell numbers and total cell spreading areas. Based on these findings, different approaches of manipulating NCD surface properties have been developed to further promote bone cell functions and subsequent tissue growth. It has been well established through experiments and computational simulations that tailoring NCD topography and nanoscale roughness (root-mean-square roughness usually <25 nm) can enhance both short- and long-term functions of osteoblast in vitro [4]. This enhancement was partly verified in vivo by implanting NCD and microcrystalline diamond (MCD) rods into rat femurs, and results showed more intense tissue growth on NCD than MCD after four and eight weeks. Modifications on NCD films by altering their surface chemistry and surface energy [examples are shown in Figures 2(c) and (d)], doping the surface with boron, and adding growth factors such as bone morphogenetic protein-2 have also demonstrated increases in osteogenic responses (e.g., adhesion, proliferation, and differentiation of osteoblasts), indicating great potential for the use of such modified NCD films in promoting bone regeneration. Similar results have been reported for mesenchymal stem cells (MSCs), where osteoinductive and osteogenic responses of MSCs are promoted on NCD compared to Ti alloys and CoCrMo. At last, because of the documented increased orthopedic implant infection rates, it is worth noting that NCD has been shown to resist Escherichia coli colonization compared to titanium and stainless steel (without the use of antibiotics) [5]. This possible anti-infection effect is highly desirable for orthopedic implant materials and, thus, should be further studied.
Nanodiamonds for Nerve Regeneration and Retinal Repair
Recently, nanodiamonds for nerve regeneration and retinal repair have attracted increasing attention because of their nanoscale feature sizes comparable to that of synapses (the specific connections between brain cells), exceptional mechanical and electrical properties, and chemical inertness. Nanodiamonds were found to perform remarkably well, serving as a platform for neuronal growth. For example, studies of neuronal growth and functions have revealed that initial cell attachment, sustained neurite outgrowth, cell-autonomous neuronal excitability, and functionality of the resulting electrical networks on nanodiamond monolayers were similar to those grown on standard protein-coated materials. These results imply that nanodiamond monolayers without protein coatings are excellent growth substrates for regenerating functional neuronal networks. Similarly, studies on the functions of neural stem cells on UNCD films in low serum conditions and without adding differentiating factors have also revealed promising results. Specifically, the hydrogen-modified UNCD can spontaneously induce stem cell proliferation, neural induction, and neuronal differentiation, suggesting the potential of using NCD films for central nervous system transplantation and neural regeneration.
For research on retinal repair, the biocompatibility of the UNCD has been evaluated both in vitro and in vivo, and a general consensus is that the NCD can be used as a robust and stable coating on implantable retinal microchips. Based on this, high-density, high-count arrays of hermetic electrical feedthroughs using conductive nitrogen-doped UNCD channels within an insulating polycrystalline diamond substrate (Figure 3) have recently been developed [6]. The biocompatible feedthroughs themselves can be used as stimulating electrodes for neural tissue, and such feedthroughs can be designed as a component of a retinal implant to restore vision to the blind. In addition, it has been already confirmed that the nitrogen-doped UNCD feedthroughs could establish efficacious and stable stimulation of retinal ganglion cells when in contact with perfused, explanted, rat retina [7]. An increasing number of studies clearly suggest the promises of using NCD films for nerve tissue simulation and repair, which brings new therapeutic opportunities to treat neural or retinal diseases.
![FIGURE 3 The SEM images of an all-diamond, hermetic electrical feedthrough array for a retinal prosthesis: (a) the inside of a feedthrough, (b) a close-up of a single feedthrough hole, (c) the structure of the external face of a feedthrough array after isolation of individual electrodes by a laser, and (d) a close-up of the N-UNCD electrode surface shown in (c). (Figure reprinted with permission from [6].)](https://www.embs.org/wp-content/uploads/2014/03/31516.png)
Nanodiamonds for Drug Delivery
Nanodiamonds can serve as highly versatile platforms for the controlled release and delivery of numerous therapeutic elements such as drugs and genes. Because a PND has extremely small and uniform sizes and is both scalable and biocompatible, it has attracted more attention for drug delivery purposes when compared to an NCD. Aqueous dispersible PND hydrogels are also able to assemble into multilayer thin films and therefore have the capability to integrate therapeutic molecules. In addition, PNDs also exhibit antioxidant, anti-inflammatory, anticarcinogenic, or antiallergic properties to some extent, which are attractive in drug delivery applications.
Owing to these attractive properties, a wide spectrum of therapeutic molecules has been loaded into PNDs for treating different diseases. In many recent studies, doxorubicin hydrochloride (Dox, a cell-death-inducing chemotherapy drug) has been successfully loaded to PNDs, and its chemotherapeutic efficacy for treating cancer has been verified in cell and animal tests. For instance, the efficacy of PND-Dox chemotherapeutics has been examined in mouse models of liver and mammary cancer. The results in both cancer models reveal that PND-Dox significantly increases cancer cell deaths and consequently inhibits tumor growth beyond conventional unmodified Dox treatment (which represents the clinical standard for most cancer treatment regimens). Some studies also suggest that PND-Dox treatment can significantly decrease the toxicity to healthy tissues compared to standard Dox treatment. In addition, researchers have also embedded PND-Dox within a parylene C polymer microfilm to achieve a stable and continuous slow release of Dox for at least one month, leading to applications for long-term or continuous treatment of cancer.
For water-insoluble drugs such as PurvalanolA (a compound for liver cancer treatment), 4-hydroxytamoxifen (an emerging drug for breast cancer treatment), and dexamethasone, which experience the problems of administration and bioavailability, PNDs have been used to enhance their water dispersibility while preserving functionality. For gene therapy, PNDs have been employed as an efficient vector for delivering genes to target sites with reduced side effects and complications. Specific genes can be immobilized on PND via linkers (e.g., low molecular weight polyethyleneimine) or an amine group that electrostatically or covalently conjugate with genes, respectively [8].
Therefore, PND-enabled drug delivery represents a promising, biocompatible, and effective strategy for achieving targeted and controlled release, and enhancing treatment efficacy and safety.
Nanodiamonds for Biodetection
![FIGURE 4 A three-dimensional tracking of a single 35-nm fluorescent PND in a live HeLa cell: (a) bright-field and epifluorescence images of the cell after PNDs (red pseudocolor) uptake and (b) 3-D reconstruction, showing the boundaries of the nucleus and the cytoplasm of the cell. The 3-D trajectory is shown in pseudocolor in (c), and the displacements of a single PND [labeled with a box in (a)] are inside the cell over a time span of 200 s. (Figure reprinted with permission from [9].)](https://www.embs.org/wp-content/uploads/2014/03/31893.png)
Besides the aforementioned unique electrical, mechanical, and biocompatibility properties, nanodiamonds can be modified to possess specific properties such as fluorescence and selective adsorption of molecules, which are of particular interests to biosensing and biodetection. The fluorescence of nanodiamonds is attributed to nitrogen–vacancy centers (a nitrogen atom next to a vacancy) and is capable of penetrating tissue, making it well suited for biological imaging and detection applications. Bright fluorescent PNDs have been used to achieve three-dimensional (3-D) tracking of a single particle within a cell (Figure 4), indicating that fluorescent PNDs are an ideal probe for long-term tracking and imaging in vivo, with good temporal and spatial resolution [9]. A recent study reported that fluorescent PNDs, in combination with fluorescence-activated cell sorting, fluorescence lifetime imaging microscopy and immunostaining, could identify transplanted lung stem/progenitor cells in vivo, and track their engraftment and regenerative capabilities with single-cell resolution [10]. The study also suggested that fluorescent PND labeling did not eliminate the self-renewal and/or differentiation properties of the cells.
The selective capture of biological molecules has also been achieved on functionalized nanodiamonds. The strategy of using functionalized PND as a high-efficiency platform for the extraction and detection of specific proteins such as aminophenylboronic acid has been developed and verified in recent studies. Selective DNA attachment on nanodiamonds has also been realized by using different linker chemistries, and such nanodiamonds can be employed as sensitive and stable substrates for DNA-based sensing. Meanwhile, attempts to create DNA-based sensors built on nanodiamonds have also been reported. For example, a type of DNA biosensor has been invented using vertically aligned diamond nanowires that are fabricated by reactive ion etching and further functionalized by genetic marker molecules.
Promises and Potentials
In the past decade, there has been a transition of nanodiamonds from structural materials that mainly provide high wear-resistance and chemical stability toward functional materials that can provide unique properties to aid in sensing, detection, and promoting tissue growth. This has been attributed to extraordinary and even unique mechanical, tribological, electrical, biocompatibility, tissue regenerative, and fluorescence properties of nanodiamonds, as well as rich opportunities for surface modification. Nanodiamonds (including PNDs and NCDs) have successfully demonstrated their promise and potential for tissue regeneration, drug delivery, and biodetection. These promises and potentials open new avenues for the detection, diagnosis, and therapy of diseases with high efficacy and safety. Although many more studies and in-depth evaluations are needed to ultimately determine the risk and safety of nanodiamonds, nanodiamonds certainly are worth more attention for numerous biomedical and health care applications.
Acknowledgements
Lei Yang would like to thank the Jiangsu Provincial Special Program of Medical Science (BL2012004), National Basic Research Program of China (973 Program, 2014CB748600), and the National 1,000 Young Talents Program of China. Thomas J. Webster would like to thank Northeastern University for funding.
References
- Y. Xing and L. M. Dai, “Nanodiamonds for nanomedicine,” Nanomed.-UK, vol. 4, no. 2, pp. 207–218, Feb. 2009.
- Q. Zhang, V. N. Mochalin, I. Neitzel, I. Y. Knoke, J. Han, C. A. Klug, J. G. Zhou, P. I. Lelkes, and Y. Gogotsi, “Fluorescent PLLA-nanodiamond composites for bone tissue engineering,” Biomaterials, vol. 32, no. 1, pp. 87–94, Jan. 2011.
- E. Pecheva, L. Pramatarova, D. Fingarova, T. Hikov, I. Dineva, Z. Karagyozova, and S. Stavrev, “Advanced materials for metal implant coatings,” J. Optoelectron. Adv. Mater., vol. 11, no. 9, pp. 1323–1326, Sept. 2009.
- L. Yang, B. W. Sheldon, and T. J. Webster, “The impact of diamond nanocrystallinity on osteoblast functions,” Biomaterials, vol. 30, no. 20, pp. 3458–3465, July 2009.
- W. Jakubowski, G. Bartosz, P. Niedzielski, W. Szymanski, and B. Walkowiak, “Nanocrystalline diamond surface is resistant to bacterial colonization,” Diam. Relat. Mater., vol. 13, no. 10, pp. 1761–1763, Oct. 2004.
- K. Ganesan, D. J. Garrett, A. Ahnood, M. N. Shivdasani, W. Tong, A. M. Turnley, K. Fox, H. Meffin, and S. Prawer, “An all-diamond, hermetic electrical feedthrough array for a retinal prosthesis,” Biomaterials, to be published.
- A. E. Hadjinicolaou, R. T. Leung, D. J. Garrett, K. Ganesan, K. Fox, D. A. Nayagam, M. N. Shivdasani, H. Meffin, M. R. Ibbotson, and S. Prawer, “Electrical stimulation of retinal ganglion cells with diamond and the development of an all diamond retinal prosthesis,” Biomaterials, vol. 33, no. 24, pp. 5812–5820, 2012.
- X.-Q. Zhang, M. Chen, R. Lam, X. Xu, E. Osawa, and D. Ho, “Polymer-functionalized nanodiamond platforms as vehicles for gene delivery,” ACS Nano, vol. 3, no. 9, pp. 2609–2616, 2009.
- Y.-R. Chang, H.-Y. Lee, K. Chen, C.-C. Chang, D.-S. Tsai, C.-C. Fu, T.-S. Lim, Y.-K. Tzeng, C.-Y. Fang, and C.-C. Han, “Mass production and dynamic imaging of fluorescent nanodiamonds,” Nature Nanotechnol., vol. 3, no. 5, pp. 284–288, 2008.
- T.-J. Wu, Y.-K. Tzeng, W.-W. Chang, C.-A. Cheng, Y. Kuo, C.-H. Chien, H.-C. Chang, and J. Yu, “Tracking the engraftment and regenerative capabilities of transplanted lung stem cells using fluorescent nanodiamonds,” Nature Nanotechnol., vol. 8, no. 9, pp. 682–689, 2013.



