To edit the human genome, you can now choose scissors or a word processor
Just four letters—A, G, T, and C—make up the alphabet of the genome. It may seem simple, but a small difference in spelling can create mutations that result in life-threatening diseases. Gene variants that cause genetic diseases come in many varieties. Transition point mutations cause conditions such as progeria, the rapid aging disease. Transversion point mutations cause sickle-cell disease and other major disorders. Small insertions can cause Tay-Sachs, which stops nerves working properly and is usually fatal, and deletions can result in cystic fibrosis.
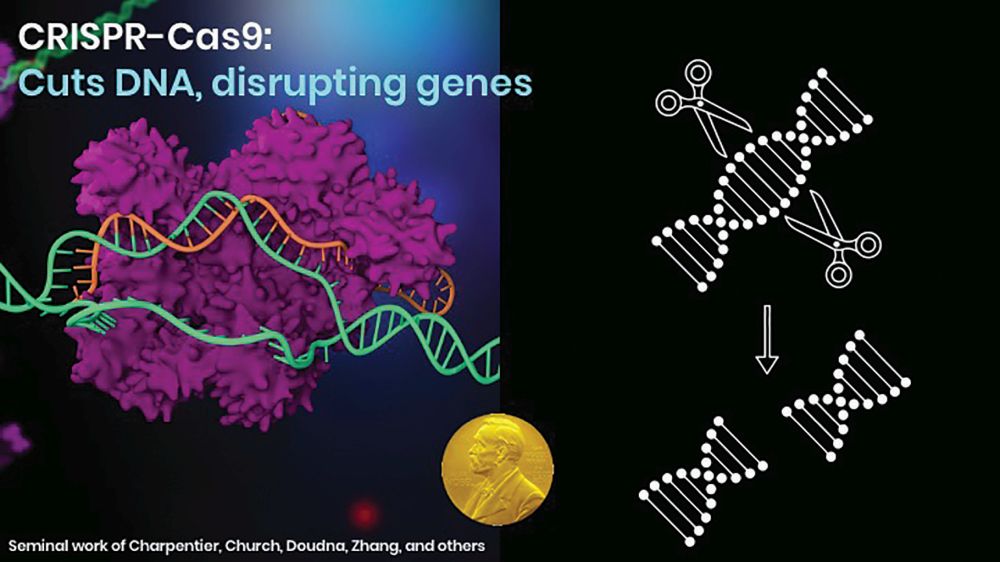
Now, tools for editing the genome can correct what could be regarded as spelling errors. The best-known is CRISPR-Cas9. This refers to clustered regularly interspaced short palindromic repeats (CRISPR), a family of DNA sequences found in DNA fragments of bacteriophages—viruses that infect and replicate within bacteria and archaea. CRISPR-associated protein 9 (Cas9) is a protein that plays a vital role in the immunological defense of certain bacteria. It enables the cutting of both strands of DNA. In the cell’s repair process, it sometimes introduces a mutation by adding or removing a single letter of DNA, or nucleotide. Using CRISPR-Cas9, this technique can turn off problematic genes or turn on silent genes—rewriting the code of life.
Two pioneers who developed the CRISPR-Cas9 genetic scissors that have enabled researchers to change the DNA of animals, plants, and micro-organisms with high precision were recently awarded a Nobel Prize. “It’s wonderful to see Jennifer Doudna and Emmanuelle Charpentier recognized with the 2020 Nobel Prize in Chemistry,” says David Liu, professor of chemistry and chemical biology at Harvard University. “The seminal Jinek et al. paper they published together has been cited more than 9500 times—an average of once every eight hours since its publication in 2012—and has helped merge the fields of CRISPR molecular biology and genome editing, yielding many subsequent discoveries and applications.”
“With new treatments for human genetic diseases already in patients, with early positive outcomes, the era of human genome editing has already begun, and Emmanuelle and Jennifer are two of the key pioneers responsible for initiating this new era,” he adds.
Changing bases
Liu, who is the Richard Merkin professor, director of the Merkin Institute of Transformative Technologies in Healthcare, and vice-chair of the Faculty at the Broad Institute of Harvard and MIT, knows a lot about innovation in CRISPR technology (Figure 1). His work on base editing and, more recently, prime editing is redefining genome editing.

CRISPR-Cas9, along with other techniques such as zinc-finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs), work through gene disruption. CRISPR-Cas9, for instance, cuts away a specific piece of DNA, allowing a new piece to be introduced into the repair process (Figure 2). Base editing, however, allows a mistake in the DNA to be repaired in place.
“A longstanding goal of the gene editing field has been the ability to install or correct all types of pathogenic mutations to study or treat the broadest possible range of the resulting diseases,” Liu remarks. “Programmable nucleases such as ZFNs, TALENs, and CRISPR-Cas9 initiated the modern era of gene editing by enabling targeted gene disruption. For most genetic diseases, however, precise target gene correction, rather than gene disruption, is needed to benefit patients.”
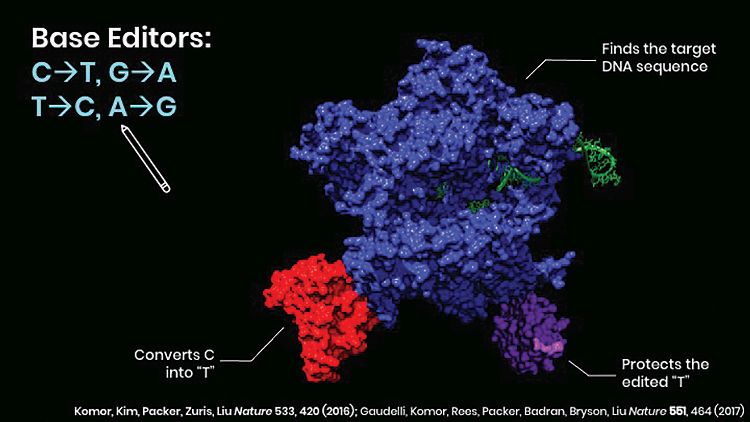
A double-strand DNA break prompts a cell to initiate end-joining repair processes that result in uncontrolled mixtures of insertions and deletions (indels), and other uncontrolled DNA rearrangements. “As a result, nearly all of the clinical trials underway using programmable nucleases are limited to the small fraction of targets for which gene disruption through indel formation can be therapeutic,” notes Liu. “As a potential solution to this challenge, we developed base editors, which use the targeting mechanism of disabled CRISPR-Cas proteins to engage a desired DNA sequence. But instead of cutting the DNA, they use deaminase enzymes from nature or evolved in our laboratory to directly convert a target base to another, then guide the cell through DNA repair processes to make this conversion permanent on both DNA strands” (Figure 3).
Cytosine base editors (CBE) convert C to T, or G to A, while adenine base editors (ABE) convert A to G, or T to C. “By converting targeted C–G base pairs to T–A, CBEs can correct up to 14% of known pathogenic point mutations,” Liu remarks. “Correcting the lion’s share of disease-associated point mutations, however, requires the opposite—conversion of an A–T base pair to a G–C base pair. In 2017, we reported the development of the ABE, which accomplishes this conversion.
”Base editing has many applications and its precision continues to improve. It can even efficiently edit mature primary cells no longer capable of undergoing cell division (mitosis) in vitro and in vivo, including in the primary cortical neurons of mice. “Since their debut four years ago, base editors have been used by many laboratories in a variety of different organisms ranging from bacteria to insects to plants to primates, resulting in hundreds of publications,” Liu remarks.
The number of CBEs and ABEs continues to grow and there is now a dizzying array of base editor choices. This can make it difficult to choose the best one for a desired edit. As a result, Liu and his colleagues have created machine learning models—known as BE-Hive—trained on experimental data from editing 38,000 genetically integrated target sites with each of 11 different CBEs and ABEs. The result is a treasure trove of data to train machine learning models that accurately predict base editing efficiency.
Unlocking the engine room door
The principle of base editing has also opened another treasure chest. Until now, mitochondrial DNA (mtDNA) remained one of the few regions that precision genome editing could not reach. Mitochondria are the body’s engine room—the structures within each cell that make energy. Mutations in mtDNA cause genetic diseases with symptoms ranging from poor growth or lack of coordination to seizures or diseases of the liver, kidney, and heart.
“The vast majority of pathogenic mtDNA mutations are transition point mutations that in principle could be corrected with a CBE or ABE,” Liu remarks. “However, CRISPR has not been successfully used in mitochondria because no mechanisms to transport guide RNAs into mitochondria are known.”
In response, Liu and Professors Joseph Mougous and Vamsi Mootha of Harvard Medical School recently developed the first precision editing capability for mitochondrial DNA. Mougous discovered that a toxic enzyme known as DddA, which is produced by the common bacterium Burkholderia cenocepacia, does not deaminate single-stranded DNA (ssDNA) or RNA, but instead deaminates cytosines in double-stranded DNA dsDNA.
Deamination is the removal of an amino group from an organic compound. It is a common process in the body, where it breaks down amino acids to release energy. Deamination reactions in DNA are essential to the rewriting of base pairs. For instance, the deamination of adenine (A) forms hypoxanthine, which pairs with cytosine (C) instead of thymine (T), resulting in transition mutation that transforms A–T into G–C.
“This raised the possibility that we could develop CRISPR-free, all-protein base editors that could enable the first precision editing of mtDNA,” Liu explains. “We made nontoxic versions of this highly toxic protein and, after testing and refining several architectures, we identified one that enables efficient targeted conversion of C–G base pairs to T–A in mitochondria or in nuclei.”
“These developments have enabled our lab and others to use base editors to treat animal models of human genetic diseases,” he adds. “So, base editors can correct pathogenic transition mutations in vitro and in vivo, in some cases with strong rescue of disease phenotype.”
Genome editing comes into its prime
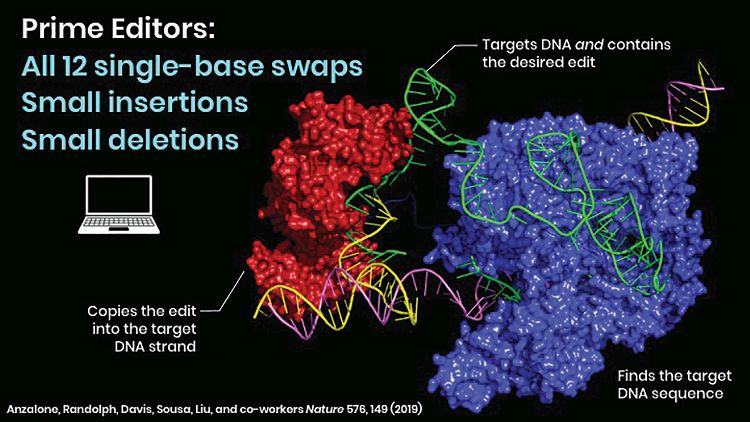
The breakthrough of base editing changed the rules of the game. Nevertheless, Liu and his colleagues did not stop their search for greater precision. If programmable nucleases such as CRISPR-Cas9 can be thought of as scissors, Liu’s next innovation—prime editing—acts more like a word processor.
Prime editing can essentially erase any base pair and replace it with another or cut and introduce long segments of DNA without breaking both DNA strands (Figure 4). This could, according to Liu, put 89% of pathogenic genetic variants within reach, though usable human therapies may still take a long time to develop. “We sought to develop other methods to directly install or correct transversions, deletions, or insertions, without requiring double-strand DNA breaks or donor DNA templates,” Liu notes. “Prime editing is a new mammalian cell gene editing technology. It uses an engineered prime editing guide RNA (pegRNA), which not only specifies the target site for editing, but also encodes the desired edit.
”So far, comparisons of the editing efficiencies of Cas9 nuclease and prime editing techniques show that prime editing offers higher or similar editing efficiencies, but greatly reduced insertions or deletions (indels) in the DNA strands.
“Base editing and prime editing offer complementary strengths,” Liu explains. “Base editors have been extensively studied and optimized for four years now, and have been used in hundreds of publications. Base editors offer higher efficiency and fewer indels, while prime editors offer greater versatility, precision, and targeting scope.”
The potential applications of both base and prime editing are still unfolding. The complex story of life is told in a language with only four letters, but errors still occur. Nevertheless, with genetic scissors and, now, word processors those errors are being made right.