In the science-fiction classic Fantastic Voyage [1], a shrink-ray zaps a submarine and the crew within it, and the resulting microscopic vehicle ventures inside a human body to destroy a blood clot and save a prominent patient’s life. While that scenario remains in the realm of make-believe, it may not be long before micro- and nanoscale robots can navigate a person’s blood vessels and execute a medical task, such as the targeted delivery of drugs or even the performance of some medical procedures.
The first step in generating these tiny medical robots is figuring out how to make them. “The artistic view is to take a large robot and just miniaturize it, but that wouldn’t work, because the force and the physics at a small scale are different,” says nanorobot pioneer Sylvain Martel, Ph.D., a professor of computer and software engineering and director of the NanoRobotics Laboratory at Polytechnique Montréal, Canada (Figure 1). For instance, as the size scale decreases, fluid viscosity increases. At the level of 3–4 nm (the diameter of the tiniest blood capillaries), a conventionally designed and propelled robot would be so bogged down it would be unable to move.

Today, several research groups are figuring out how to build controllable robots to traverse these extremely small spaces and their non-Newtonian fluid environments. Martel’s group is one that has collaborated with experts in fields such as computer science, engineering, microbiology, and clinical medicine—to name a few—to develop what he calls a “natural nanorobot” that can navigate the body’s microvasculature and interstitial spaces to a chosen destination.
Medical Nanorobots
Martel participated in the Massachusetts Institute of Technology’s NanoWalker project, which, in 1999, assembled a three-legged robot that was about as big as a grape and could take thousands of nanoscale-sized steps per second [2]. This bolstered his interest in nanotechnology and robotics, and, once he started his own lab in Montréal in 2001, he began considering medical applications. He felt one area that could especially benefit was the conveyance of cancer drugs.
“I saw that a lot of money and research were going toward the development of different therapeutic agents, but not much was being done on the delivery side,” he recalls. “That’s why my lab has been doing this for the last 16 years—we’re trying to bring a drug directly from point A to point B, where point A is the injection site and point B is the region where the drug has its maximum efficacy.”
Targeted drug delivery is a worthy hurdle to jump because, with current systemic chemotherapy, “only 0.7% of chemotherapy drugs go to the region where they can have some therapeutic effect,” Martel explains. “The rest goes throughout your body, where it causes secondary effects; the more effective the cancer drug is, the more toxic it is, so this is a big problem.” Recent advances (using PEGylation) have increased the circulation time of cancer drugs so that up to 2% of the drugs can make it to the tumor site, he adds, but that is still nowhere near good enough.
“What we engineers are trying to do with the robotics route is minimize the circulation time of drugs, and we can do that if we have a delivery system that knows where to go,” Martel says. “We aren’t developing new drugs. What we are doing is just taking the fantastic cancer drugs that others are making, and we are developing a transport system so the drugs get to the location where they can do their job effectively and without affecting the patient.”
Microbes to the Rescue
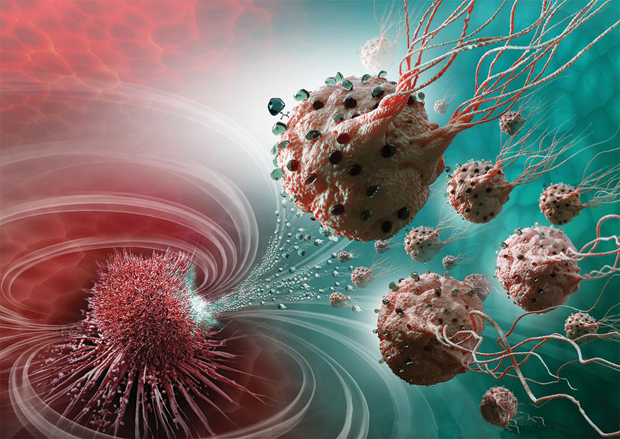
Instead of fabricating a vessel to carry the cancer-fighting drugs, Martel and his extended research group are seeking a solution from nature and, in particular, from magnetotactic bacteria (bacteria that orient using Earth’s magnetic field). To each bacterium, they attach 70 drug-containing liposomes, or molecular “bags” of drugs. They then inject a large population of loaded bacteria into the body and employ a weak, computer-controlled magnetic field to herd the bacteria toward the tumor, where each bacterium’s oxygen sensors kick in to guide it to regions of low-oxygen concentrations (or hypoxic zones) created by the active cancer cells. Once on-site, the liposomes dissolve, which releases the drug at what would otherwise be a hard-to-reach and difficult-to-treat region of the tumor (Figure 2). What happens to the bacteria? They are nonpathogenic and die off within approximately 30 minutes.

Central to this project are the bacteria, specifically the MC-1 strain of Magnetococcus marinus. In nature, they live in the deep sea, where the oxygen concentration is only 0.5%, which happens to match the oxygen concentration in the active region of metastatic tumors. This region, the tumor’s hypoxic zone, is low in oxygen because the tumor burns large quantities during the process of cell duplication. The bacteria have evolved a sensory system to detect their preferred hypoxic habitat, and they also have a propulsion system—their flagella—that allows them to move through the syrupy microfluid environment toward that low-oxygen area, explains project collaborator Gerald Batist, M.D. (Figure 3, right). Batist is director of the McGill Center for Translational Research in Cancer based at the Jewish General Hospital in Montréal.
“By taking advantage of these bacteria’s capabilities, we showed that our nanorobots could carry the chemotherapy—in this case, an active metabolite of a drug called irinotecan—exactly to the hypoxic area, and we could kill the tumor cells. This was the first time anyone had shown preferential killing of hypoxiczone tumors,” Batist reports. The research group conducted this work on colorectal cancer cells in mice and reported its findings in 2016 [3].
According to Martel, these natural nano – robots carry drugs to the hypoxic zone with an initial efficiency of 55%. “The remaining 45% of the drugs go into systemic circulation. But, because at least 55% is going to the proper site, that allows the use of (lesser amounts of) drugs from the beginning,” he says.
“The implications are enormous,” Batist remarks. “Even when a tumor has a tremendous response to current chemotherapy strategies, some cells often survive, and these cells are frequently tumor stem cells harbored in the hypoxic zone of the tumor. The delivery of drugs to this zone has been a ‘holy grail,’ and now with (these natural nanorobots), we can do it.” He adds, “This is something that will sensitize tumors to a variety of treatments, including immunotherapy, and have broad effects.”
The research group is taking the nanorobots into a preclinical, tumor-bearing model within roughly a year, and, if all goes as expected, Batist estimates human clinical trials will begin by late 2019. “We’ve been working for years on ways to block resistance, and this jumps over all of those efforts and gets the drugs right to the resistant cells by mechanical delivery. I think this will be a game-changer for clinical practice.”
Big Tasks for Tiny Robots
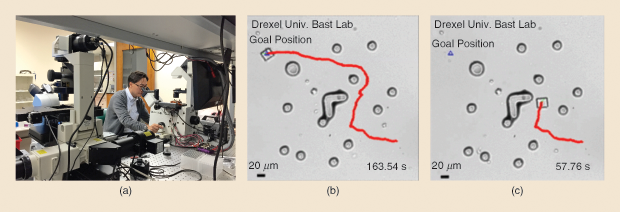
Another robotics expert who is working on tiny robots is Min Jun Kim, Robert C. Womack Endowed Chair Professor of Engineering at Southern Methodist University (SMU) in Dallas, Texas. In 2011, while he was an engineering professor and director of the Biological Actuation, Sensing, and Transport Lab at Drexel University in Philadelphia, Kim’s team showed that an electrical field could be used to move a simple robot (Figure 4) made up of a microfabricated inorganic structure coated with the common pathogen Serratia marcescens, a naturally negatively charged microbe [4]. He and his research group at SMU have now demonstrated [5] that the field can direct the bacteria-enveloped miniature robot to a chosen destination while avoiding obstacles along the way (Figure 5).
To accomplish this, Kim’s group devised a steering algorithm that takes advantage of the slight electrical variations caused by the obstacles themselves. The algorithm detects the distortions and passes along that information to the bacteria, which then autonomously make the appropriate course modifications to dodge the obstacles. Kim has shown similar success using other external control signals, such as magnetic fields, chemical gradients, and optical signals including ultraviolet and infrared light. “So, overall, the power of the microorganism steers this structure, and, by introducing external stimuli, we can control their motion,” he explains.
In another project, Kim and his research group are learning how to control living cells. Here, they internalize iron oxide nanoparticles into motile protozoa, such as the common freshwater species Tetrahymena pyriformis. Through the external application of electrical and magnetic fields, they can then control these single-celled organisms [6].
This work illustrates how one common external force can control large numbers of microrobots [7], [8]. Such capabilities are especially important if the robots are to perform a complex task that involves the use of a tool. For instance, a tool might enable the penetration of a tumor cell membrane for the selective release of a drug inside the cell, grasping of cells for cell-manipulation purposes, or building of scaffolding for tissue engineering.
Building and Using Microtools
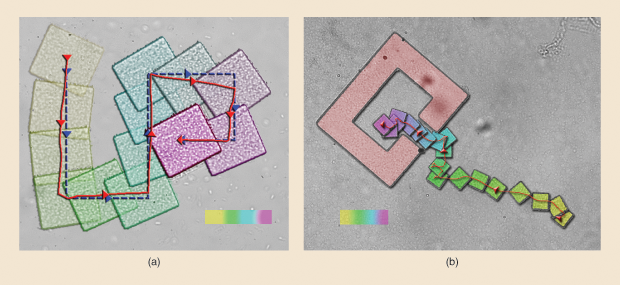
One tactic for developing tools is to introduce microrobots that are essentially controllable building-block particles. Once they reach a chosen location in the microfluidic environment, a magnetic signal or external stimulus instructs them to construct the tool. Kim is collaborating with Aaron Becker, assistant professor of electrical and computer engineering at the University of Houston, to orchestrate large-scale tool assembly (Figure 6). They reported early success in 2017 with the publication of a joint paper in IEEE Robotics and Automation Letters [9]. There, they showed they could apply a uniform, global, external force—in this case, a magnetic field—that caused a swarm of simple two-dimensional particles, called polyominoes, not only to successfully navigate a microfluidic environment and reach a destination, but also to self-assemble into a two-dimensional structure.
Becker is especially interested in using magnetism to trigger a release of mechanical energy. For instance, a signal might cause a tool to tunnel through a cell membrane for direct and very localized delivery of drugs to that cell and only that cell. One of his projects he calls a “magnetic hammer,” which is basically a tube with a small ball inside. Using the very weak magnetic forces produced by the gradient coils of a magnetic resonance imager (MRI), the researchers can cause the ball to resonate by bouncing back and forth inside the tube. “On one side of the tube, we’ve got an impact plate, and on the other side, we’ve got a spring; so it bounces off one side and hammers on the other,” Becker explains, likening it to a pile driver’s action. “In our paper, which I’m excited to report was just accepted in IEEE Robotics and Automation Letters [10], we demonstrated that the hammer could push through brain tissue.”
In another project, Becker’s research group is using self-assembly to trigger the delivery of energy. “What we can do—and we have videos of this on YouTube [11]—is steer each component using the magnetic force of the MRI, but when we bring the pieces close together and they link up, we can have a targeted release of mechanical energy that’s been stored. It’s called a Gauss gun,” he says. He likens it to the autoinjector that nurses now use to take a blood sample. “A nurse will push this little plastic autoinjector up next to your finger, and then he or she pushes a button that actually shoots the needle into the skin. It takes very little force to push the button, but there’s enough stored energy to pop that needle (through the skin).”
At this point, Becker’s hammer and Gauss gun are in the milliscale range, and he is currently working out the physics of developing similar tools for the microscale, microfluidic environment. The latter will open doors to a wide range of medical uses. “One of the first things we’re looking at is cyst fenestration. If we could deliver an impulsive force at the right place, we could puncture a cyst that is blocking a channel,” he says. “Another application we’re considering is the implantation of seed particles, such as brachytherapy seeds, to the precise location you want and without having to do even a minimally invasive surgery. We could do the same with the placement of stem-cell therapy.”
Teamwork Makes the Dream Work
The whole idea of microrobots and nanorobots is new territory, and “new” is a hard sell in medicine, Becker observes. “We’re arguing for a way of doing treatment and surgery that hasn’t been done before, and in the medical community, where a really cool idea can also be a very dangerous idea, it will take a winning story of a working treatment for this field to really be accepted.”
Whether the first “winning story” is the nanobot-led destruction of a patient’s metastatic tumor or a clot-biting tool à la Fantastic Voyage, one thing is clear: a large team of engineers, clinicians, and scientists from many backgrounds will have been involved in the path of development.
Martel’s project has faced numerous hurdles, and over the years he has brought in dozens of experts to jump them: chemists to work out the details of SN-38 attachment to the bacteria, computer scientists to generate algorithms to adjust the magnetic field and control the bacteria, lab technicians to handle the mice, medical doctors to ensure that the nanorobots actually fulfill the medical needs, and many others. “Sometimes I stop and think, ‘Wait a minute. How many people do we want in this thing?’” he says, laughing. But that doesn’t keep him from adding more. In fact, he is now developing natural nanorobots for prostate cancer and working on a drug-delivery technique that will bypass the blood–brain barrier, both of which add more complexity and require more experts.
“I have to say, the most difficult part of these projects is keeping the team alive, but if it was just engineers, we couldn’t have done it. With a project this complex, we had to have a multidisciplinary team,” Martel asserts.
Kim agrees. “This field is completely interdisciplinary and multidisciplinary. A single group cannot do everything by itself. That’s why I’m working with electrical engineers, fluid mechanicians, microbiologists, biochemists, and others,” he notes.
Collaborating with so many other people can be arduous, especially at first, but it is also exceedingly rewarding, Batist insists. “As you can imagine, we had engineers, medical chemists, clinicians, and biologists all trying to talk to each other, so it was like a Tower of Babel at the beginning. But once we started to understand each other and began to use one another’s phrases, it became very exciting to everyone,” he recalls.
This kind of cross-disciplinary exchange is not limited to the collaborators within one research group. “In one sense, we’re all working as individual groups to see how fast we can do things ourselves, but we’re also sharing advice and data with other groups, and even sharing people,” Becker says, noting that he is sending one of his postdoctoral researchers to Montréal to meet with Martel. “There is amazing camaraderie in this field, and it is helping to move everyone along.”
Batist adds, “Breakthroughs are at the interfaces of disciplines. By working together, we challenge ourselves to think outside the box, and that’s fantastic.”
References
- Fantastic Voyage. California: Harry Kleiner, Twentieth Century Fox Film Corporation; 1966.
- S. M. Martel, P. G. Madden, L. Sosnowski, and I. W. Hunter, “NanoWalker: A fully autonomous highly integrated miniature robot for nanoscale measurements,” in Proc. SPIE 3825, Microsystems Metrology and Inspection, vol. 111, Sept. 15, 1999. doi:10.1117/12.364292.
- O. Felfoul, M. Mohammadi, S. Taherkhani, D. de Lanauze, Y. Z. Xu, D. Loghin, S. Essa, S. Jancik, D. Houle, M. Lafleur, L. Gaboury, M. Tabrizian, N. Kaou, M. Atkin, T. Vuong, G. Batist, N. Beauchemin, D. Radzioch, and S. Martel, “Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions,” Nature Nanotechnology, vol. 11, no. 11, pp. 941–947, Nov. 2016.
- E. B. Steager, M. S. Sakar, D. H. Kim, V. Kumar, G. Pappas, and M. J. Kim, “Electrokinetic and optical control of bacterial microrobots,” IEEE J. Micromech. Microeng., vol. 21, no. 3, pp. 035001, 2011.
- H. Kim and M. J. Kim, “Electric field control of bacteria-powered microrobots (BPMs) using static obstacle avoidance algorithm,” IEEE Trans. Robot., vol. 32, no. 1, pp. 125–137, 2016.
- D. H. Kim, U. K. Cheang, L. Kohidai, D. Y. Byun, and M. J. Kim, “Artificial magnotactic motion control of Tetrahymena pyriformis using ferromagnetic nanoparticles–A tool to fabricate new class of microbiorobots,” IEEE Appl. Phys. Lett., vol. 97, pp. 173702, 2010.
- P. S. S. Kim, A. Becker, Y. Ou, A. A. Julius, and M. J. Kim, “Imparting magnetic dipole heterogeneity to internalized iron oxide nanoparticles for microorganism swarm control,” J. Nanopart. Res., vol. 17, no. 3, pp. 144, Mar. 2015.
- U. K. Cheang, F. Meshkati, H. Kim, K. Lee, H. C. Fu, and M. J. Kim, “Versatile microrobotics using simple modular subunits,” Sci. Rep., vol. 6, pp. 30472, 2016.
- S. Manzoor, S. Sheckman, J. Lonsford, H. Kim, M. J. Kim, and A. T. Becker, “Parallel self-assembly of polyominoes under uniform control inputs,” IEEE Robot. Autom. Lett., vol. 2, no. 4, pp. 2040–2047, Oct. 2017.
- J. Leclerc, A. Ramakrishnan, N. V. Tsekos, and A. T. Becker, “Magnetic hammer actuation for tissue penetration using a millirobot,” IEEE Robot. Autom. Lett., vol. 3, no. 1, pp. 403–410, Jan. 2018.
- J. Leclerc, A. Ramakrishnan, N. V. Tsekos, and A. T. Becker. (2017, Aug. 1). Magnetic hammer milli-robot for tissue penetration. [Online].