Although asthma has been around since Hippocrates’ time, more people are being diagnosed with the disease than ever before. Over the last 20 years, the global burden of asthma has increased by almost 30%, as more than 235 million people—most of them children—cope with the breathlessness and wheezing characteristic of the disease. In particular, cases have spiked in China and India, where pollution is reported to sometimes be deadly. Researchers with the Health Effects Institute, a Boston-based nonprofit that studies the health effects of pollution, recently reported that air pollution in India and China alone contributed to more than half of the four million deaths worldwide due to air pollution in 2015.
The Face of Asthma
With asthma, a patient’s airways become inflamed, which causes coughing, breathlessness, and wheezing. “The inflammation in asthmatics is systemic,” says Chunrong Lin, an asthma researcher at Stanford University, California. “But the lungs are just the organs in which that inflammation manifests.”
Asthma has no cure, but patients can manage their condition using inhalers, generally the first line of treatment. These devices are typically loaded with drugs such as bronchodilators and corticosteroids to relax the muscles that constrict the airways and reduce inflammation in the lungs.
But, for a disease like asthma, patients who receive drugs through a respiratory route could potentially see no benefit at all if they’re not using their inhalers correctly or as frequently as they should. For inhalers currently on the market to be the most efficacious, both patients and healthcare professionals need a significant amount of training.

“Adherence [to inhaled therapy for asthma] is driven by so many different factors,” says Jeff Weers, the chief technology officer of Respira Therapeutics, a pharmaceutical company based in Albuquerque, New Mexico, focused on treating pulmonary conditions (Figure 1, right). “There are simpler situations where folks simply forget to take their medication, but there are also more complex scenarios where people start taking their medication, start feeling better, and then think they don’t need it anymore.” In either scenario, patients likely do not derive the maximum possible benefit from their medication.
Today, companies and researchers are working to devise inhalers that are less dependent on patient usage and can deliver the drugs asthmatics need more consistently. In recent years, researchers have also begun developing pills and injections to manage more severe forms of the disease, and other groups have designed wearable devices to track respiratory patterns and patient adherence as a way to prevent the onset of an asthma attack.
Simplify the Design
Inhalers come in all shapes and sizes, but the two most commonly used are pressurized metered-dose inhalers (pMDIs) and dry powder inhalers. Most pMDIs are boot-shaped and deliver medication mixed with a propellant in a spray. To use these, patients typically need to first shake the inhaler well and then remove the cap and inhale, while applying pressure to dispense the medication. Dry powder inhalers are handheld, disk-shaped devices, typically having a dose indicator to keep a count of the number of doses used. Dry powder inhalers contain only the medication without an additional propellant.
Weers, who has worked on asthma therapeutics for nearly two decades, says that one of the major current trends in the industry is simplifying the device. Additionally, he says, “If we can make devices that just require three steps to be used—open, inhale, close—you can eliminate a lot of steps [where] people typically make mistakes.”
For instance, some children use the DISKUS inhaler (a flat, round dry powder inhaler from Advair). To make sure the inhaler will deliver the dose, the patient first needs to press a bar to ready the device. However, if those inhalers had a cap that, once opened, would do a number of things to prepare the inhaler for use—thus bypassing the need for the patient to press a bar—this new device could reduce one step and so the potential for error.
Recently, GlaxoSmithKline, a pharmaceutical company headquartered in London, has developed the BREO Ellipta, a DISKUS-mode inhaler that can deliver medicine consistently in just three steps: opening the inhaler, inhaling, and closing the device. However, opening the inhaler pierces the blister that contains the dose; if the patient doesn’t inhale immediately thereafter and replace the cap, he or she loses a dose.
Researchers are looking at ways to incorporate breath actuators into inhalers. Currently, pMDIs tend to generate aerosol faster than the patient can inhale. Breath-actuated inhalers would release the medicine when the patient breathes in, ensuring better-timed drug delivery.
Improving Aerosol Delivery to Lungs
Because each person breathes differently, it’s imperative to have a drug-device combination that will deliver the optimal dose to the lungs independently of how patients inhale.
Currently, there’s great variability of dosing based on how patients breathe. If they inhale passively, very little of the drug in the inhaler might actually reach their lungs. If patients inhale sharply, a larger dose of the drug could be dispersed. Moreover, current devices also deposit a significant fraction of the inhaled dose in the oral cavity and trachea instead of the lungs. These off-target effects are undesirable, and improved inhalers would ideally divert more of the aerosol treatment directly to the lungs.

To treat asthma and other lung conditions, smaller particles are often better because they can more readily traverse the narrow airways and reach their intended sites of action. Richard Dalby, a professor of pharmacy at the University of Maryland (Figure 2, right), is looking at ways to dissolve drugs into the propellant of pMDIs. Dalby is investigating the use of pressure to break up larger particles of drugs into smaller ones that can ultimately have a greater therapeutic effect in the lungs. This approach is in experimental phases and has yet to be commercialized.
Other researchers are looking at ways to optimize the interactions among carrier molecules often formulated in asthma medications. The most widely used inactive ingredient in pMDIs is lactose, which is ideal given its smooth surface and favorable shape. Hugh Smyth, a pharmaceutical scientist with the University of Texas at Austin, has worked on changing the size of lactose particles and investigating this approach in terms of device performance. His research team has found that much larger lactose particles typically improved the efficiency of delivery. This approach has been patented but not yet commercialized.
In addition, combinations of drugs within inhalers could compromise delivery of both medicines in the correct ratios. For instance, if the patient doesn’t properly shake the inhaler container, drug particles will either sink to the bottom or float to the top. Ultimately, the patient would take in a nonhomogenous mixture. “If you can put both drugs in a single porous particle, that fixes the ratios of the two drugs,” Dalby reasons.
Already, researchers have started using porous particles, typically made up of biodegradable polymers, to help deliver the drugs used in pMDIs. For instance, AstraZeneca has developed Bevespi Aerosphere, an inhalation aerosol in a pMDI, which uses the company’s Co-Suspension Technology involving porous, low-density phospholipid particles that help homogenize the distribution of the drug throughout the lungs.
Previously, when Weer was at Novartis Pharmaceuticals, he patented PulmoSphere, a light and porous dry powder for the delivery of asthma medications that became commercially available in 2013. Made up of small phospholipid-based particles, PulmoSphere (Figure 3) reduced the likelihood of the actual drugs sticking to each other. “We’ve figured out how to bypass most of the deposition [of the drug] in the mouth and throat, which leads to dramatic decreases in the variability of drug delivery in patients,” says Weer. “That, to me, is the key to getting everything you need in terms of low variability and high delivery, low side effects and high efficacy, and, hopefully, fewer exacerbations [as reported by patients].”
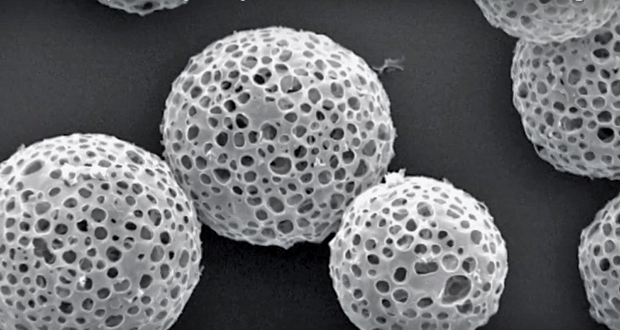
Researchers at Pearl Therapeutics, a pharmaceutical company in Redwood City, California, that focuses on treating and preventing respiratory diseases, have also generated a way to increase deposition by creating engineered porous phospholipid microparticles and suspending them in 1,1,1,2-Tetrafluoroethane, a propellant. Their approach has since been incorporated into the company’s proprietary platform.
Smart and Connected Inhalers
Currently, patients do not necessarily always have a reliable, foolproof way of knowing whether or not they have taken their dose. Patients who press the inhaler to dispense a dose but don’t actually receive one derive no medical benefit at all. If a patient’s inhaler has a counter but the counter does not advance by one after the patient takes a dose, then the patient could think he or she has more of the drug left than actually exists. And asthmatics who use pMDIs often cannot see how much medication they have remaining; drugs are typically deposited in small amounts at a time and kept in metal canisters inside the inhaler.
Consequently, research teams and companies are now developing inhalers with electronics in the mouthpiece that can count drug doses based on drug discharge rather than surrogates that can be related to discharge. These so-called smart inhalers could also remind patients when they need to take a dose, coach a patient in how to use the inhaler properly, or pull information from the inhaler to a database and make it available for physicians to view their patients’ inhaler usage.
Already, Adherium, a digital health technology company based in Auckland, New Zealand, has developed the Smartinhaler, a device that tracks when medications are taken, reminds patients to take their medications, and uploads all usage information via Bluetooth when the device is within range of a compatible phone or computer application. According to a study of 220 children who used the Smartinhaler platform, adherence to medication nearly doubled over the course of four years.
According to Weers, “There’s no device that has it all”—in other words, there is no inhaler that combines a simplified three-step process, breath-actuated dose counters, engineered particles to optimize drug delivery, and smart technology capabilities. Currently, Respira is developing one device that has a three-step feature and uses engineered particles to make the drug delivery process independent of how a patient inhales, thereby taking out the error and variability of therapies.
Still, access to these futuristic inhalers is not necessarily guaranteed. In the United States, insurance companies act as gatekeepers for inhalers, as virtually no patients pay for them out of pocket. Therefore, insurance companies would have to weigh the benefits of these new devices (and the increased costs involved) against the possible hospitalization costs associated with taking care of chronically nonadherent asthmatics. In addition, the next generation of inhalers may not reach the international market. For instance, in China and India, asthmatics typically buy their inhalers from local manufacturers—and it’s questionable how those locally made devices compare with the technologies available in the West.
Wearables

In addition to inhalers, many other research teams and corporations are designing monitors—all in various stages of development—to measure whether patients are adhering to their treatment regimen. Ultimately, the goal of such monitors is to improve management of the disease.
Many traditional monitoring devices that measure patient adherence can be quite large, are expensive to manufacture and commercialize, and must be plugged into an electrical source to operate. But now researchers are taking steps to make these devices smaller and more compatible for use with inhalers.
GlaxoSmithKline has recently partnered with Propeller Health, a company based in Madison, Wisconsin, that manufacturers monitors. These digital sensors, which record how frequently someone is taking his or her medication, are mounted to an inhaler and send the data back to a user’s phone through an app (Figure 4). These sensors could conceivably help make sure that patients are taking their medications.
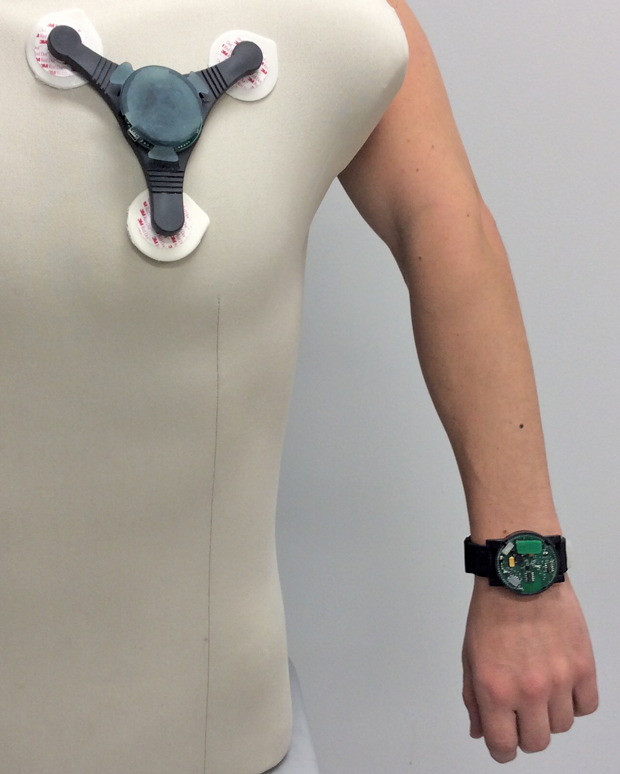


Researchers at North Carolina State University have developed wristbands and patches as wearables that can track parameters such as movement, heart and respiratory rate, the amount of oxygen in the blood, and wheezing in the lungs, among other metrics (Figures 5 and 6). The sensors also detect contaminants in the air, such as volatile organic compounds and ozone. The monitors use a self-powered spirometer to measure lung function. Data from these sensors get transmitted wirelessly to a computer for collection and recording by customized software. Currently, the North Carolina State researchers are collecting data from patients in the clinic as they fine-tune the device, according to Alper Bozkurt, an engineer at the university (Figure 7, below).
Scientists have also developed asthma monitors that can precisely measure the concentration of nitric oxide (NO). Because NO is often produced in response to inflammation, patients with asthma typically have higher levels of NO in a single exhale, compared to their nonasthmatic counterparts. Therefore, it’s important to have tools that can accurately and quickly detect NO levels.

When she was a professor at the State University of New York at Stony Brook, Perena Gouma, a materials engineer now at the University of Texas at Arlington, started work on a new asthma monitor that could detect NO at low parts-per-billion concentrations using polymorphic metal oxides. More recently, scientists at Rutgers University in New Brunswick, New Jersey, have begun developing a graphene-based sensor that could aid in the early detection of asthma attacks or help patients manage other respiratory diseases (Figure 8). “Graphene is biofriendly and resilient to biofouling and corrosion, which makes it great for complex biological matrices like exhaled breath, and it’s also very sensitive,” says Mehdi Javanmard, an engineer at Rutgers (Figure 9). Similar to the monitors developed by Gouma and her colleagues, these graphene sensors detect for levels of nitrite, a by-product of NO metabolism in the body.
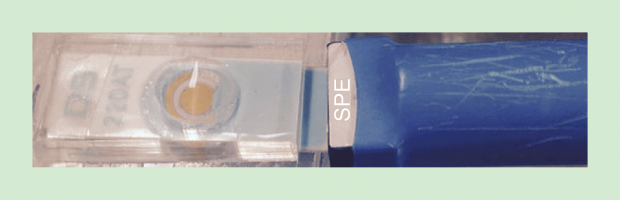
Nongjian Tao, an engineer at Arizona State University, Tempe, is commercializing a handheld asthma sensor with ZCube, a research venture of the Italian pharmaceutical company Zambon (Figure 10, below). This rechargeable sensor can detect relevant exhaled markers in real time and so help doctors adjust a patient’s medication dosage; if combined with global positioning system technology, the device can also track where patients are when using an inhaler.

Biologics
Although many patients with asthma use inhalers and are responsive to standard corticosteroid-bronchodilator treatment, approximately 5–10% of people do not respond to conventional therapies. Clinicians view these severe cases as a major problem. Therefore, researchers are also looking into the development of biologics, which target the cells and specific pathways that cause other methods of inflammation associated with asthma. These types of therapies have the potential to personalize future asthma treatment.
Biologics for the treatment of asthma that have been approved by the U.S. Food and Drug Administration (FDA) are few and far between. Currently, Omalizumab (sold as Xolair) is one injection for asthma that has been FDA-approved for use in the United States. It’s delivered once every two or four weeks in patients six years and older who do not respond to conventional corticosteroids. The drug itself is an antibody targeting human immunoglobulin E, a molecule involved in the inflammation response in patients with asthma.
In 2015, the FDA also approved Nucula (mepolizumab), developed by GlaxoSmithKline, to manage asthma in patients 12 years and older who suffer from severe attacks and do not respond to conventional combinations of corticosteroids and bronchodilators. Delivered as an injection once every month, Nucula is an antibody against interleukin-5 (IL-5), a protein that controls the presence of a type of white blood cells (known as blood eosinophils) that cause airway damage in response to inflammation.
Novartis, the Swiss pharmaceutical company, is developing the pill Fevipiprant, which users can take daily to control their asthma. The active compound, an antibody, targets the prostaglandin D2 receptor 2, a protein involved in allergic inflammation. Currently, the drug is being evaluated for its pharmacokinetic properties in patients in the United States.
Even as pharmaceutical companies are developing antibodies for the treatment of asthma, other researchers are investigating the potential of different drug targets, especially to treat more severe forms of asthma. Blood eosinophils are particularly abundant in people who suffer from more severe forms of asthma. Even after inhaled corticosteroid treatment, these cell types persist. Therefore, scientists are looking to target the proteins and pathways involved in the eosinophilic asthma response, such as the receptor for IL-5, the agents that target IL-4 and IL-13, and thymic stromal lymphopoietin.
Neutrophils, another type of white blood cells, are also found in the airways of patients with asthma. Their presence is believed to release proteins and other factors that prolong asthma symptoms, often leading to a more severe phenotype. In addition, scientists are investigating antibodies—such as brodalumab—that can neutralize cytokines, such as IL-17A and IL-17F, that are important in activating neutrophils.
Despite all these advances in designing new drugs for asthma, the price point may be hefty. According to Chunrong Lin of Stanford, a single shot of the new biologics in development can run up to US$2,000, and they could cost patients thousands of dollars annually. Moreover, these new therapies may have effects that researchers can’t yet predict. “We don’t know what the downstream effects of these new therapies are yet—until we use them more frequently and in a larger population,” Lin says. “What we do know is that [these new drugs] are pretty safe and effective.”
References
- J. Weers and T. Tarara, “The PulmoSphere platform for pulmonary drug delivery,” Ther. Deliv., vol. 5, no. 3, pp. 277–295, Mar. 2014.
- A. H. Chan, A. W. Stewart, J. Harrison, C. A. Camargo, Jr, P. N. Black, and E. A. Mitchell, “The effect of an electronic monitoring device with audiovisual reminder function on adherence to inhaled corticosteroids and school attendance in children with asthma: A randomized controlled trial,” Lancet Respir. Med., vol. 3, no. 3, pp. 210–219, Mar. 2015.
- J. Dieffenderfer, H. Goodell, S. Mills, M. McKnight, S. Yao, F. Lin, E. Beppler, B. Bent, B. Lee, V. Misra, Y. Zhu, O. Oralkan, J. Strohmaier, J. Muth, D. Peden, and A. Bozkurt, “Low-power wearable systems for continuous monitoring of environment and health for chronic respiratory disease,” IEEE J. Biomed. Health Inform., vol. 20, no. 5, pp. 1251–1264, Sept. 2016.
- A. Gholizadeh, D. Voiry, C. Weisel, A. Gow, R. Laumbach, H. Kipen, M. Chhowalla, and M. Javanmard, “Toward point-of-care management of chronic respiratory conditions: Electrochemical sensing of nitrite content in exhaled breath condensate using reduced grapheme oxide,” Microsyst. Nanoeng., vol. 3, p. 17022, May 2017.
- G. Garcia, C. Taillé, P. Laveneziana, A. Bourdin, P. Chanez, and M. Humbert, “Anti-interleuekin- therapy in severe asthma,” Eur. Respir. Rev., vol. 22, no. 129, pp. 251–257, Sept. 2013.
- S. K. Özdemir and S. Bavbek, “Prospects for new and emerging therapeutics in severe asthma: The role of biologics,” Expert Rev. Respir. Med., vol. 11, no. 6, pp. 505–512, May 2017.



