Magnetic resonance imaging (MRI) is the preferred modality for soft tissue imaging because of its nonionizing radiation and lack of contrast agent. Due to interactions between the MR system and active implantable medical devices (AIMDs), patients with implants such as pacemakers are generally denied access to MRI, which presents a detriment to that population. It has been estimated that 50–75% of patients with a cardiac device were denied access to MRI scanning and, moreover, that 17% of pacemaker patients need an MRI within 12 months of implantation [1]. In recent years, AIMD manufacturers, such as Biotronik, have assessed the conditional safety of devices in MRI.
To mitigate the associated risks, AIMD manufacturers, together with academics, MR manufacturers, and regulatory scientists, have developed an international technical specification to assess MR conditional safety of AIMDs [2]. The MR conditional safety of a device is then assessed in a laboratory by generating conservative estimates of each field component, often exceeding what is physically possible in a commercial MR system. Virtual humans (VHs) are used for evaluating several Date of publication: 14 July 2017 hazards. Of these, radio-frequency (RF)-induced heating of the tissue surrounding the AIMD is the topic of our discussion here.
Evaluating RF Heating with VHs
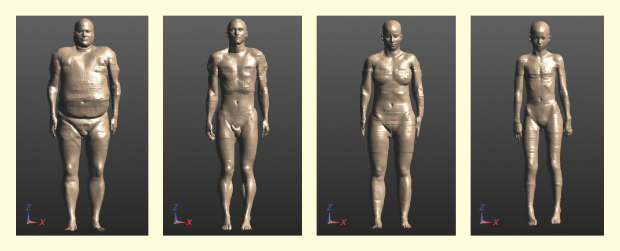
Many variables contribute to RF-induced heating in cardiac leads: clinical trials alone cannot sufficiently stress an AIMD to the low-probability, high-risk conditions possible in an MRI environment. Most combinations of patient, device, lead, and MRI scanner induce only a modest amount of heating; virtually all patients in any clinical trial of reasonable size will experience such modest amounts of heating. We are interested, however, in examining the tails of the induced heating distribution. Modeling is the tool that allows us to efficiently complete this examination. The most widely accepted VHs for this article are the Virtual Population family from the Foundation for Research on Information Technologies in Society (IT’IS); four of these models are shown in Figure 1.
Figure 2 provides a flowchart of the RF-induced heating hazard evaluation process, illustrating the integration of VHs in practice. This process follows the transfer function method for developing a lead model, in which heating at the lead tip is related to the incident field. Modeling is used to generate three-dimensional (3-D) spatial electronic fields (e-fields) in multiple VHs and a variety of scanners and positions (Figure 3).
The power of using VHs becomes evident as one realizes that various bodies, scanners, and positions can be evaluated quickly and efficiently. These generated e-fields are combined with a separately derived model for each lead–device combination, and an induced energy is reported for every lead pathway in those models (Figure 4). A cumulative distribution of induced powers is produced, and a target energy (incorporating a device-based risk analysis) is identified for final evaluation. Animal studies determine the ultimate safety of the target energy, with the pacing capture threshold (PCT) increase used as a proxy for thermal tissue damage [3].
Why Use VHs?
The use of VHs provides a direct benefit for patient access to MRI, as millions of simulations of any combinations of patients and leads, device orientations, MR system technology, and so forth are easily evaluated [4].
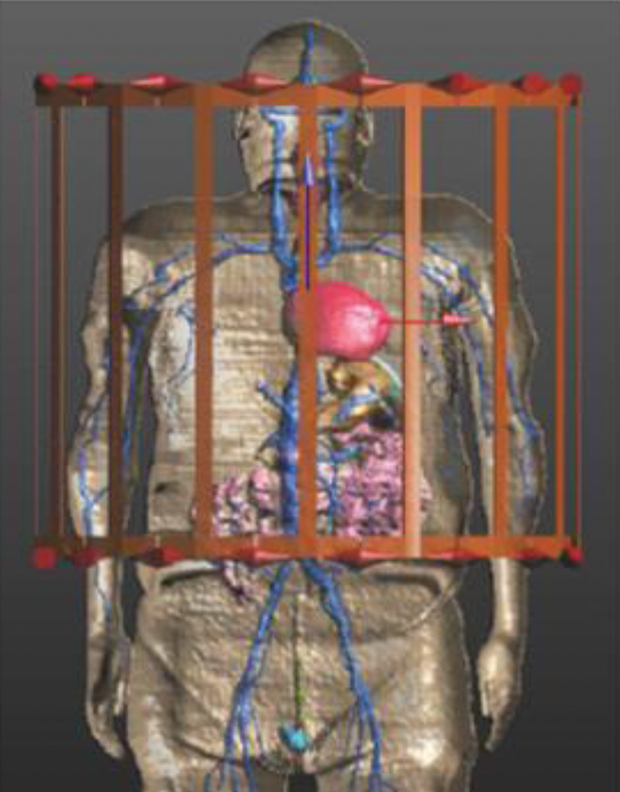
A library of multiple VHs spanning the population in terms of body mass index (BMI) can be used in a safety assessment; this is much easier than finding human subjects within specific size categories for a clinical trial. Moreover, obtaining clinical data is expensive and time consuming; numerical modeling provides a rapid and low-cost way to retrieve in vivo parameters, including those that are difficult or impossible to measure in a clinical setting. A human body greatly disturbs the MRI bore’s electromagnetic fields, producing multiple local specific absorption rate (SAR)/thermal hot spots (Figure 5). Therefore, VHs provide a means of studying the impact of these deviations. In the future, modeling could extend to cosimulation of electromagnetic and physiological phenomena such as PCT changes from RF heating.
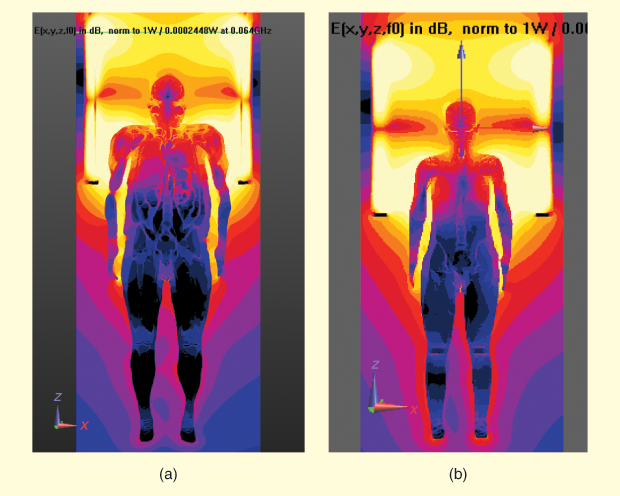
The patient’s impact on the MR system is also readily investigated using modeling. To study statistically extreme cases, multiple body positions inside the bore, including the supine and prone positons, are investigated, as changing the position of the body within the bore presents a change in load to the coil. As MRI manufacturers also build various coil dimensions, the variability between body positions can be significant. In 3-Tesla systems, each manufacturer uses its own shimming technique to improve field homogeneity. Computational modeling has proved a very convenient way to rapidly evaluate any shimming condition [5]. It is much more efficient to evaluate radiation limits such as those for whole-body SAR, head SAR, and peak field levels with modeling for different body and body position scenarios. Conservative results are obtained by modeling at these peak field levels. MRI scanning is often done below these levels.
Through the use of VHs, the effect of device orientation, including the lead pathway, can be thoroughly assessed. For example, the impact of lead pathway variability can be studied in isolation; in a clinical setting, the lead pathway cannot be varied within a single patient. Further, using VHs to model device behavior allows one to make comparative statements—a given solution to reduce lead heating can be evaluated against a baseline under precisely the same conditions. Using VHs also adds the ability to gauge the MR conditional safety of device prototypes, as device variants can be modeled much more quickly than they can be manufactured, significantly shortening device design cycles.
The development of VHs is an enabling technology for MR conditional safety assessments, as the high risk associated with low-probability events is impractical or unethical to pursue using clinical trials. Current VHs were developed for use with the finite-difference time-domain method. In the future, VH models that can be used with any computational technique will allow optimal selection of the numerical method based on the device geometry. Finally, VHs should be developed that are validated for both physiological and electromagnetic modeling, to perform virtual clinical trials to demonstrate MR conditional safety.
References
- R. Kalin and M. S. Stanton, “Current clinical issues for MRI scanning of pacemaker and defibrillator patients,” Pacing Clin. Electrophysiol., vol. 28, no. 4, pp. 326–328, Apr. 2005.
- “Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device,” International Organization for Standardization, Geneva, Switzerland, Tech. Spec. ISO/TS 10974:2012(E), May 2012.
- B. L. Wilkoff, T. Albert, M. Lazebnik, S.-M. Park, J. Edmonson, B. Herberg, J. Golnitz, S. Wixon, J. Peltier, H. Yoon, S. Willey, and Y. Safriel, “Safe magnetic resonance imaging scanning of patients with cardiac rhythm devices: A role for computer modeling,” Heart Rhythm, vol. 10, no. 12, pp. 1815–1821, Dec. 2013.
- J. E. Brown, R. Qiang, P. J. Stadnik, L. J. Stotts, and J. A. Von Arx, “MR conditional safety assessment of implanted medical devices: Advantages of computational human phantoms,” in Proc. 38th Annu. Int. Conf. IEEE Engineering in Medicine and Biology Society, Orlando, FL, 2016, pp. 6465 –6468.
- T. S. Ibrahim, R. Lee, B. A. Baertlein, A. Kangarlu, and P.-M. L. Robitaille, “Application of finite difference time domain method for the design of birdcage RF head coils using multi-port excitations,” Magn. Reson. Imag., vol. 18, no. 6, pp. 733–742, 2000.



