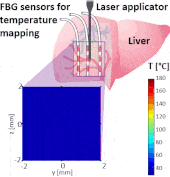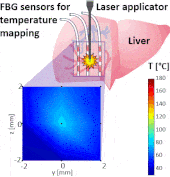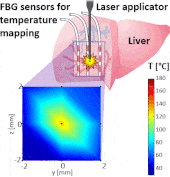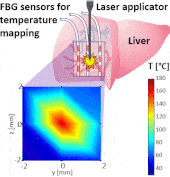
Thermo ablative treatments (like microwave, radiofrequency and laser ablation) are gaining relevance as viable minimally invasive treatment for cancer therapy. The current challenge in their use is to achieve a balance between complete destruction of malignant cells and safeguarding of the surrounding healthy tissue.
So far, blood perfusion plays an important role for thermal ablation treatment success, especially in the case of highly vascularized organs like liver. Accordingly, in this work an experimental analysis of the blood perfusion effects on the temperature distribution during laser ablation has been performed. To mimic reality, blood perfusion within the ex-vivo swine liver was simulated using artificial vessels and heated water flow inside them.
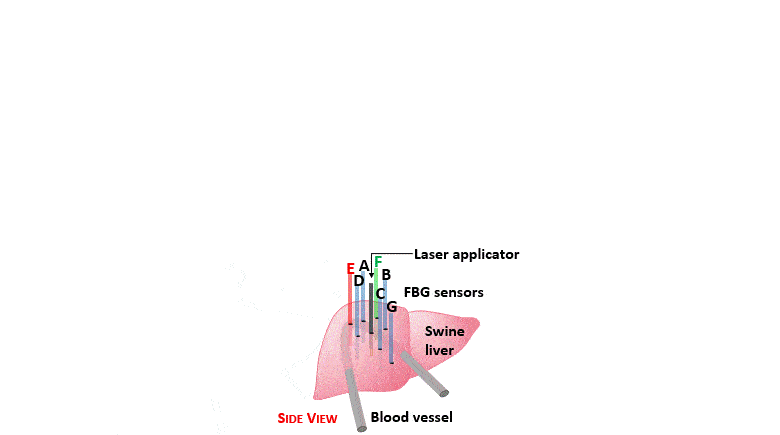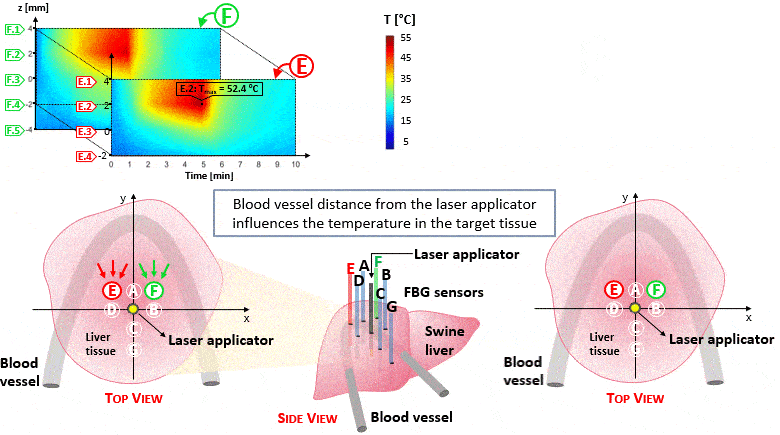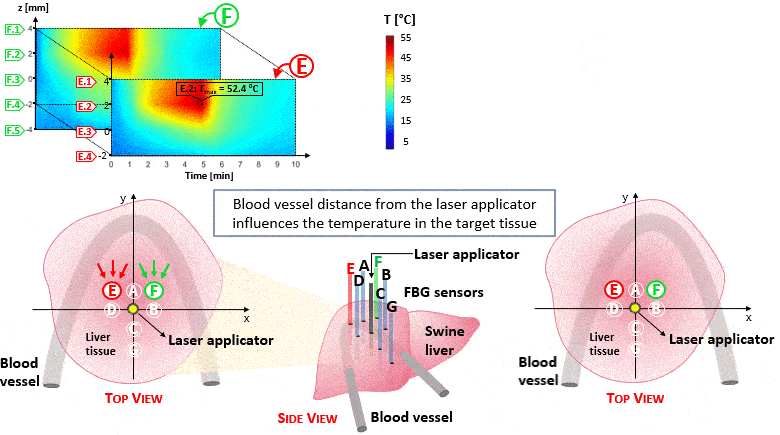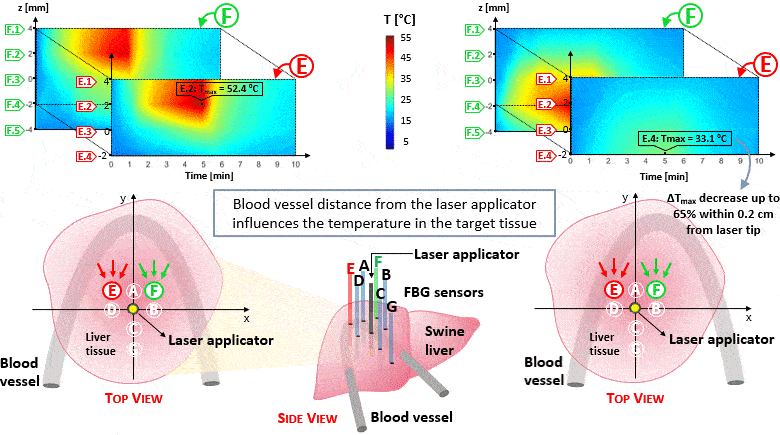
The setup proposed to assess these effects is based on several arrays of Fiber Bragg Grating sensors (FBGs) placed around the laser applicator, allowing a real-time temperature monitoring with a resolution of 0.1 °C and a spatial resolution around 1 mm.
The relative positions of the FBGs with respect to the vessels are used to quantify the vessel influence on the thermal gradient by investigating a symmetric and an asymmetric configuration of sensors, vessels and laser applicator.
The used setup permitted to accurately measure the heat propagation and to observe a relevant tissue cooling near to the vessel up to 65%.
This result could be useful for clinical applications because tissue cooling by perfusion can affect the size of the produced ablation lesion, thus the estimation of the blood perfusion impact on the thermal map reconstruction could be essential in the achievement of a complete necrosis of the target tissue and clinically relevant.