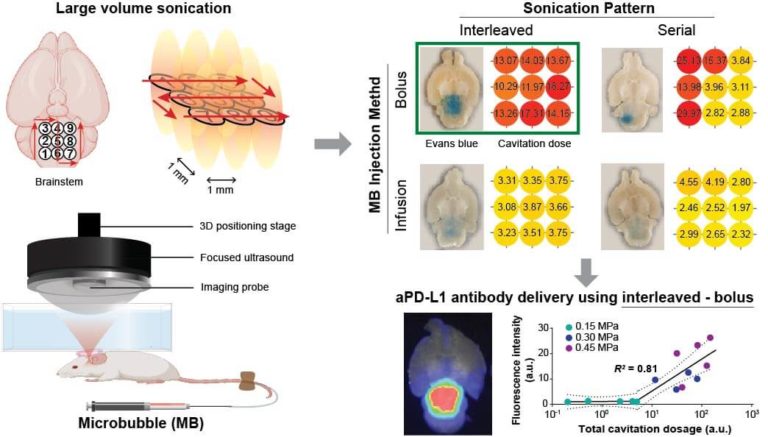
Diffuse intrinsic pontine glioma (DIPG) is the deadliest pediatric brainstem tumor, with challenges of lacking effective therapeutic drug delivery due to the intact blood-brain barrier (BBB). Focused ultrasound combined with microbubble-mediated BBB opening (FUS-BBBO) has been shown feasible to achieve large-volume drug delivery to the brainstem in both preclinical and clinical studies. This study aims to compare different treatment strategies for achieving efficient and homogeneous large-volume delivery of an immune checkpoint inhibitor (aPD-L1).
We identified multiple-point sonication pattern (interleaved or serial) and microbubble administration method (bolus or infusion) as two critical parameters for large-volume FUS-BBBO in mice. We first compared the four combinations of these parameters on the efficiency and homogeneity of FUS-BBBO outcome at mouse brainstem using Evan blue, a model drug, as readout. The delivery outcome was quantified using fluorescence imaging. The strategy which gave the best outcome was selected to deliver fluorescently labeled aPD-L1 at different sonication pressures. For all treatment, we acquired 2D passive cavitation imaging (PCI) to monitor the large-volume sonication.
Interleaved sonication combined with bolus injection of microbubbles (interleaved – bolus) achieved the highest efficiency and spatial homogeneity of the large-volume Evans blue extravasation compared to the other strategies. Interleaved – bolus was also identified as the optimal strategy by the cavitation dose quantified from PCI. By applying interleaved – bolus to aPD-L1 delivery at different sonication pressures, we found a strong segmented linear correlation (R2 = 0.81) between the total cavitation dose and the total fluorescence intensity of delivered aPD-L1. This study suggests that efficient and homogeneous large-volume FUS-BBBO can be achieved using interleaved – bolus and monitored by PCI.