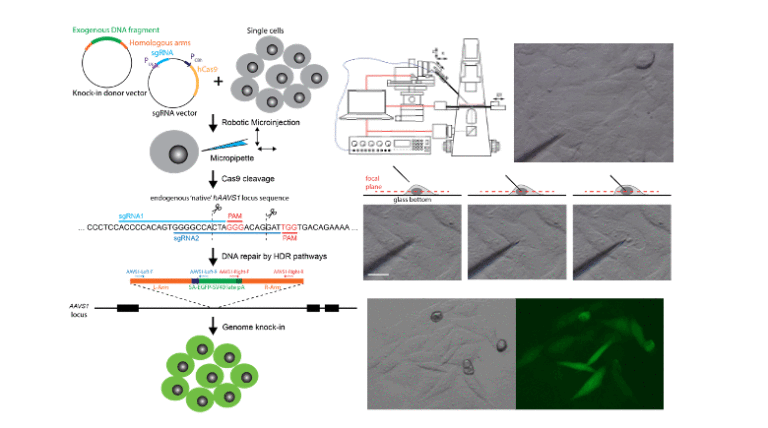
Gene therapy with engineered nucleases offers promising solutions for genetic disorders by editing DNA sequences or modulating gene expression. The non-viral delivery of the prokaryotic clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 (Cas9) nuclease system provides promising solutions for gene therapy. However, chemical and physical delivery approaches for gene knock-in are confronted by significant challenges to overcoming the drawbacks of low efficiency and high toxicity. This work aims to use of the high productivity microinjection system to perform targeted gene insertion studies. The robotic microinjection can deliver various macromolecules into cell cytoplasm or nucleus efficiently.
We demonstrate that delivering CRISPR/Cas9 plasmids using a precise, high-throughput robotic microinjection system can efficiently generate enhanced green fluorescent protein (GFP) knock-in cells. Two overlapping sgRNAs are constructed for targeting human AAV virus site 1 (AAVS1) loci, and the GFP donner template flanked by two homology arms is designed to achieve effective gene knock-in. By using the robotic microinjection system, direct delivery of plasmid encoding designed gRNA and CRISPR nuclease sequence mixed with donor template plasmid into HepG2 cells can achieve eGFP knock-in cells with an efficiency of 41%. Sequence and biochemical analyses indicate that the eGFP knock-in cells exhibit no detectable changes at potential off-target sites.
A case study of injecting genome-modified cancer cells into zebrafish (Danio rerio) embryos is conducted to establish an in vivo cancer model. The knock-in of eGFP into HepG2 cells can enable the visualization of a tumor based on eGFP fluorescence signals. The successful demonstration of eGFP insertion into HepG2 cells using robotic microinjection will explore a new avenue for facilitating the fast establishment of in vivo cancer models for personalized treatment.