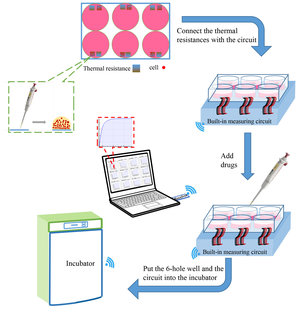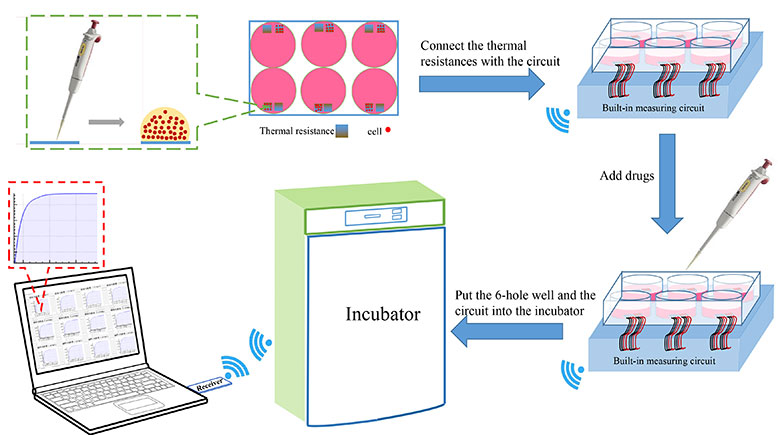
It is vitally necessary to measure the cellular temperature to fully understand life sciences. A method allowing to measure the cellular temperature for a normal growing state without doing damage to the cells and disturbing their intercellular communication is needed. Here, a wireless, real-time, high-throughput temperature detection method is developed. The acquisition system applies a high-precision reference resistor and a low real-time measurement current (below or equal to 0.14 mA) to reduce self-heating via the intermittent measurement. Cells of a small volume cell medium are cultured on the surface of the platinum thermal resistor and subsequently measured in the incubator. The resistance resolution of the circuit exhibits 20 mΩ, which corresponds to no more than 0.01 °C. The resistance deviations of each channel are corrected with software compensation. The linearity between the temperature and resistance of the sensors lies above 0.999 in the applied temperature range (30 °C–42 °C). Observations with the scanning electron microscope (SEM) show that the cells grow well on the sensor surface. The latter is composed of a glass glaze, which is nontoxic for organisms. The cell population temperature measurements under norepinephrine action present an obvious temperature rise, which can be the result of the drug binding to the receptors on cell membrane thus promoting a cationic inflow. The platinum sensor and multi-channel acquisition system can be used to determine the temperature changes of cells in their original state. The wireless, real-time, high-throughput temperature detection method is particularly suitable to evaluate the thermogenic ability of growing cells that interact with other matter or organisms. The proposed method can help to explore thermal changes in cell populations, intercellular connections, and social connections of cells.